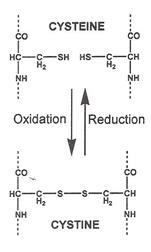
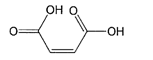
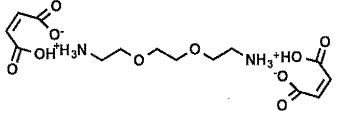
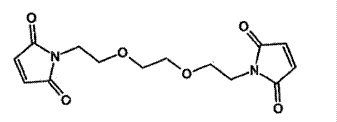
B e f o r e :
LORD JUSTICE DAVIS
LORD JUSTICE McCOMBE
and
LORD JUSTICE ARNOLD
____________________
Between:
| |
(1) L'OREAL (U.K.) LIMITED
(2) L'OREAL SA
|
Appellants
|
| |
- and -
|
|
| |
(1) LIQWD INC
(2) OLAPLEX, LLC
|
Respondents
|
____________________
Justin Turner QC and Mark Chacksfield QC (instructed by Baker & McKenzie LLP) for the Appellants
Iain Purvis QC and Katherine Moggridge (instructed by Hogan Lovells LLP) for the Respondents
Hearing dates: 5-6 November 2019
____________________
HTML VERSION OF JUDGMENT APPROVED�
____________________
Crown Copyright ©