- This is an appeal by the Appellants (“MGI”) from an order of Birss J (as he then was) dated 18 February 2021 declaring, for the reasons given in his judgment dated 20 January 2021 [2021] EWHC 57 (Pat), that four patents owned by the Respondent (“Illumina”) were, as amended, valid and had been infringed by MGI. The judge also held that a fifth patent was invalid, but there is no appeal by Illumina against his order for revocation of that patent.
- The four patents which are the subject of the appeal fall into two groups:
i) European Patent (UK) No. 1 530 578, European Patent (UK) No. 3 002 289 and European Patent (UK) No. 3 587 433 (“the Modified Nucleotide Patents”). These three patents are divisionals. The first two are entitled “Modified Nucleotides for Polynucleotide Sequencing” and the third is entitled “Modified Nucleotides”. They are based on an application filed on 22 August 2003. Although the earliest claimed priority is from a US application filed on 23 August 2002, Illumina only sought to maintain priority from the second priority document, a British application filed on 23 December 2002 (“P2”). It is common ground that the issues in relation to the Modified Nucleotide Patents may be determined by reference to the second of the three patents (“289”).
ii) EP (UK) No 2 021 415 entitled “Dye Compounds and the Use of Their Labelled Conjugates” (“the 415 Patent”). The application for this patent was filed on 16 May 2007 claiming priority from a US application filed on 18 May 2006.
- The patents describe and claim inventions in the field of DNA sequencing, more specifically a technique known as “sequencing by synthesis”. The inventions described and claimed in the Modified Nucleotide Patents are concerned with using an azidomethyl group (-CH2N3) as a reversible chain terminator in sequencing by synthesis. The 415 Patent describes and claims a class of molecules useful in sequencing by synthesis consisting of three moieties: a nucleotide, a particular cleavable linker and a particular fluorescent dye.
- The judge was faced with a considerable number of issues. On the appeal, only three of those issues are still live, namely:
i) Are the Modified Nucleotide Patents obvious over S. Zavgorodny et al, “1-Alkythioalkylation of Nucleoside Hydroxyl Functions and Its Synthetic Applications: A New Versatile Method in Nucleoside Chemistry”, Tetrahedron Letters, 32 (15), 7593-7596 (1991) (“Zavgorodny”)?
ii) Are the Modified Nucleotide Patents entitled to priority from P2? It is common ground that, if the Modified Nucleotide Patents are not entitled to priority from P2, then they are obvious over Illumina’s US Patent Application No. 2003/0104437 (“Barnes”), which was published on 5 June 2003.
iii) Is the 415 Patent obvious as a collocation of non-inventive features?
The Modified Nucleotide Patents
The skilled team
- There was a substantial dispute before the judge as to the identity and attributes of the person or team skilled in the art to whom the Modified Nucleotide Patents were addressed. The judge found at [96] and [98] that they were addressed to a team working on research into sequencing by synthesis with two members. One member would have a background in molecular biology or genetics, with a focus on DNA sequencing in particular, the other member would have a background in organic chemistry. They would both have a post-graduate degree, probably a PhD but perhaps a Masters, and some years’ research experience.
Common general knowledge
- There was also a substantial dispute as to the skilled team’s common general knowledge. Before turning to the judge’s findings, it may assist comprehension if I give a couple of paragraphs of explanation based on the primer agreed between the parties for trial.
- The structure of DNA. DNA consists of two complementary strands that wind around one another to form a double helix. The strands of DNA are made up of a string of individual nucleotides, which are composed of deoxyribose (a 5-carbon sugar), a nitrogenous base, and a phosphate group, as shown below. There are four different nucleotides in DNA, which differ from each other by their nitrogenous bases: adenine (A), cytosine (C), guanine (G), and thymine (T). Adenine and guanine are “purine” bases, whereas cytosine and thymine are “pyrimidine” bases.
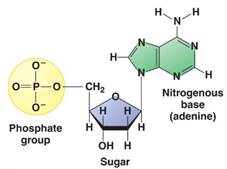
- The nucleotides in each strand are linked by their phosphate group, which connects the 5' carbon atom (the atom in the -CH2- group external to the ring) of one deoxyribose to the 3' carbon atom (the atom with a hydroxyl (-OH) substituent) of the deoxyribose of the next nucleotide, to form a sugar-phosphate backbone as shown below. The two complementary strands assemble together by pairing of the nitrogenous bases mediated by complementary hydrogen bonds, such that C pairs with G and A pairs with T.

- Sanger sequencing. In the 1970s two ways of sequencing DNA were devised, each named after their inventors: Maxam-Gilbert sequencing and Sanger sequencing. Maxam-Gilbert sequencing was based on cutting the DNA strands using reagents which break the sequence at known places and analysing the results to deduce the original sequence. Sanger sequencing supplanted Maxam-Gilbert sequencing, and automated machines running Sanger sequencing were used in the human genome project in the 1990s.
- The judge explained Sanger sequencing as follows:
“40. … Starting from the double stranded DNA of interest, a single strand is taken and used as a template in the method. DNA polymerase is used to add complementary nucleotide bases to the single template strand, one at a time. The complementarity of DNA means that the particular nucleotide base added at a given stage by the polymerase enzyme will be determined by the template sequence. So if the relevant nucleotide in the template is G then a C will be added to the growing complementary strand …. The trick to Sanger sequencing is that the free nucleotides to be added are not in their natural form.
41. Natural nucleotides have the capacity to form chains by joining together. … The chemical group at the 3' position is a hydroxyl (OH) group and the group at the 5' position is a triphosphate ester. The two ends connect together to form a link in the chain called a phosphodiester bond, liberating a molecule called pyrophosphate. A single strand of DNA therefore consists of a chain of these nucleotides and will have a ‘3' end’ at one end of the chain and a ‘5' end’ at the other end of the chain.
42. The way natural DNA synthesis works is that when a new nucleotide is added to the complementary strand, its 5' end is linked to the 3' end of the existing nucleotide which was already present. Once incorporated the unused 3' end of that newly linked nucleotide is ready to connect to the next fresh nucleotide, and so the complementary chain will grow.
44. So in Sanger sequencing when a ddNTP is added, the chain cannot grow any further. Since there are four nucleotides (C, G, A and T) one can make four mixtures whereby each mixture has all four of C, G, A and T nucleotides in it but in each mixture, some examples of one kind of nucleotide are in the blocked ddNTP form instead of the natural dNTP form. Therefore when the DNA polymerase incorporates a ddNTP into the newly synthesised DNA strand, synthesis of the strand ceases (i.e. chain termination occurs). The Sanger sequencing process therefore results in the synthesis of a large number of copies of the template strand, which are terminated at random lengths according to the position at which a ddNTP is incorporated. A population of DNA strands of different lengths is therefore obtained, which end either with A, T, G, or C. These DNA strands of different lengths are then resolved using manual or automatic approaches and the DNA sequence can be understood. In a manual version of the process radiolabelled ddNTPs are used and the resulting radiolabelled copies of the template strand are size separated by gel electrophoresis. The DNA sequence of the template strand is determined from the order of the bands in the gel, as shown below:
 ”
”
- Sequencing by synthesis. Sanger sequencing had certain drawbacks. The judge found that, by December 2002, there were a number of teams researching alternatives to Sanger sequencing. These included techniques which one would now call sequencing by synthesis. Only one such technique had been demonstrated to work, a technique called pyrosequencing. The details of pyrosequencing do not matter for present purposes. It suffices to say that it was different to sequencing by synthesis using reversible chain termination.
- Reversible chain termination. As the judge explained, well before 2002, some in the art had the idea of trying to do a variant of Sanger sequencing, but with a chain terminator which was reversible. Such terminators are also referred to as blocking groups, protecting groups and protective groups. The idea was that, if the blocking of the 3' hydroxyl group could be reversed after the identity of the added nucleotide had been confirmed, then the next nucleotide in the chain could then be added and the process repeated.
- Two research groups led this field. The first was a group led by Richard Gibbs (Baylor College of Medicine) and Kevin Burgess (Texas A & M University). This group published five papers on reversible chain termination in the period from 1994 to 1999, including Michael Metzker et al, “Termination of DNA synthesis by novel 3'-modified deoxyribonucleoside 5'-triphosphates”, Nucleic Acids Research, 22(2), 4259-4267 (1994) (“Metzker 1994”). The second was a group at the Pasteur Institute in Paris led by Bruno Canard and Robert Sarfati. This group published six papers from 1994 to 1999, including Canard and Sarfati, “DNA polymerase fluorescent substrates with reversible 3′-tags”, Gene, 148(1), 1-6 (1994) (“Canard 1994”). Neither group had published anything on this topic between 1999 and 23 December 2002.
- The judge’s findings as to the state of the skilled team’s common general knowledge concerning reversible chain termination began at [104] as follows:
“ … the common general knowledge would also include knowledge of the concept of reversible chain termination. A fair number of papers related to reversible chain terminators had been published before the priority date. As a matter of the common general knowledge of a sequencing by synthesis skilled team, the team would know that there was a body of papers and know how to find them. I would hold that unprompted, their common general knowledge would include knowledge of the existence of two particular groups who had published experimental results in more than one paper. They were the Gibbs/Burgess group and the [Canard/]Sarfati group. Again, unprompted, the common general knowledge would include the existence of two particular papers, which were frequently cited. They are Metzker 1994 and Canard 1994. This is not a finding that any particular content in either paper was common general knowledge. What was common general knowledge was that these papers existed, published results and represented the farthest anyone had got with reversible chain terminators as a concept.”
- The judge considered Metzker 1994 at [106]-[110]. In summary, he found that it disclosed a single cycle of incorporation of a 3'-O-protected nucleotide in which the protective group was a 2-nitrobenzyl group, deprotection and re-initiation of DNA synthesis in which a natural dNTP was incorporated. The judge considered Canard 1994 at [111], and found that it also disclosed a single cycle of incorporation of a 3'-O-protected nucleotide using a different protective group.
- The judge’s findings as to the common general knowledge continued as follows:
“120. …. By 2002 ... the view of the skilled [team] …. was that neither Metzker nor Canard had achieved anything more than an initial incorporation in 1994, and their later efforts up to 1999 had not succeeded.
121. What was the common general knowledge about the problems which had to be solved? MGI’s characterisation of the ‘problem to be solved’ was the identification of a reversible chain terminator which could meet the requirements for use in sequencing by synthesis. A critical question is whether that problem or something like it was part of the common general knowledge of the skilled [team]. The reason this is critical is that the evidence of [MGI’s expert witness] Prof Marx that the invention was obvious was based on an approach made clear in his evidence and cross-examination. His approach was that the skilled [team] looks at Zavgorodny with the specific aim in mind of finding a blocking group [they] might be able to use in a reversible chain terminator sequencing process. Moreover the questions put to [Illumina’s expert witness] Prof Leadlay were on essentially the same premise (that the skilled [team] came to the cited art interested in taking forward sequencing by synthesis with a new reversible chain terminator).
122. Illumina submitted that the idea of pursuing new chemical groups as reversible chain terminators on the 3' end of the nucleotide in sequencing by synthesis was not representative of the common general knowledge at 2002. I agree. My reasons are as follows.
123. First, the skilled [team] did not lack chemical groups to try as protecting groups. The Greene & Wuts textbook [Theodora Greene and Peter Wuts, Protective Groups in Organic Synthesis, 3rd edition (John Wiley & Sons, 1999)] illustrates that.
126. Overall, in my judgment the common general knowledge of the skilled [team] in 2002 was that they knew of the concept of sequencing by synthesis with reversible chain terminators, but they also knew that it had not succeeded in practice. The skilled [team] also understood that to make it work one needed to come up with a system in which one could repeatably incorporate nucleotides linked to specific labels one at a time in a reversible way, but they did not know with any degree of specificity what particular problem or problems had to be solved so as to take this forward. It may well have been that the technique simply could not be made to work. The attitude of the skilled [team] in 2002 was not an upbeat one.”
- Synthetic organic chemistry. The judge found that the skilled team’s common general knowledge included two aspects of synthetic organic chemistry:
“128. … First … the idea of a reversible protecting group in organic chemistry is very well established. They find utility throughout organic chemistry as [a] way of protecting one functional group in a molecule from reacting so that changes can be made elsewhere. The Greene & Wuts textbook which has already been mentioned is a large encyclopaedia of possible protecting groups. It includes azides.
The Modified Nucleotide Patents
- As noted above, it is common ground that 289 may be taken as representative of the Modified Nucleotide Patents. The specification of 289 is a moderately lengthy and detailed one running to 147 paragraphs, but much of the detail is unimportant for present purposes.
- The specification explains at [0001] that the invention relates to modified nucleotides having a removable protecting group, their use in polynucleotide sequencing methods and a method for chemical deprotection of the protecting group.
- It describes the concept of sequencing by synthesis at [0004]:
“Sequencing by synthesis of DNA ideally requires the controlled (i.e. one at a time) incorporation of the correct complementary nucleotide opposite the oligonucleotide being sequenced. This allows for accurate sequencing by adding nucleotides in multiple cycles as each nucleotide residue is sequenced one at a time, thus preventing an uncontrolled series of incorporations occurring. The incorporated nucleotide is read using an appropriate label attached thereto before removal of the label moiety and the subsequent next round of sequencing. In order to ensure only a single incorporation occurs, a structural modification (‘blocking group’) of the sequencing nucleotides is required to ensure a single nucleotide incorporation but which then prevents any further nucleotide incorporation into the polynucleotide chain. The blocking group must then be removable, under reaction conditions which do not interfere with the integrity of the DNA being sequenced. The sequencing cycle can then continue with the incorporation of the next blocked, labelled nucleotide. In order to be of practical use, the entire process should consist of high yielding, highly specific chemical and enzymatic steps to facilitate multiple cycles of sequencing.”
- It identifies what is required of a blocking group for this purpose at [0005]:
“To be useful in DNA sequencing, nucleotide, and more usually nucleotide triphosphates, generally require a 3'OH-blocking group so as to prevent the polymerase used to incorporate it into a polynucleotide chain from continuing to replicate once the base on the nucleotide is added. There are many limitations on the suitability of a molecule as a blocking group. It must be such that it prevents additional nucleotide molecules from being added to the polynucleotide chain whilst simultaneously being easily removable from the sugar moiety without causing damage to the polynucleotide chain. Furthermore, the modified nucleotide must be tolerated by the polymerase or other appropriate enzyme used to incorporate it into the polynucleotide chain. The ideal blocking group will therefore exhibit long term stability, be efficiently incorporated by the polymerase enzyme, cause total blocking of secondary or further incorporation and have the ability to be removed under mild conditions that do not cause damage to the polynucleotide structure, preferably under aqueous conditions. These stringent requirements are formidable obstacles to the design and synthesis of the requisite modified nucleotides.”
- It identifies the problem addressed by the invention at [0006]:
“Reversible blocking groups for this purpose have been described previously but none of them generally meet the above criteria for polynucleotide, e.g. DNA-compatible, chemistry.”
- At [0007] Metzker 1994 is acknowledged, but it is said that “the 3'allyl blocked compound was not used to demonstrate a complete cycle of termination, deprotection and reinitiation of DNA synthesis”. At [0011] it is said that Metzker 1994 does not “actually teach[…] the deprotection of 3'-allylated hydroxyl group in the context of a sequencing protocol”. The specification goes on:
“… Whilst the use of an allyl group as a hydroxyl protecting group is well known - it is easy to introduce and is stable across the whole pH range and to elevated temperatures - there is to date, no concrete embodiment of the successful cleavage of a 3'-allyl group under DNA compatible conditions, i.e. conditions under which the integrity of the DNA is not wholly or partially destroyed. In other words, it has not been possible hitherto to conduct DNA sequencing using 3'OH allyl-blocked nucleotides.”
- The specification introduces the invention at [0013]:
“The present invention is based on the surprising development of a number of reversible blocking groups and methods of deprotecting them under DNA compatible conditions. Some of these blocking groups are novel per se; others have been disclosed in the prior art but, as noted above, it has not proved possible to utilised these blocking groups in DNA sequencing.”
- At [0015] there is a consistory paragraph identifying a first aspect of the invention as a modified nucleotide or nucleoside molecule having a removable 3'-OH blocking group covalently attached thereto, such that the 3' carbon atom has attached a group of the structure -O-Z wherein Z is defined as encompassing a substantial range of substituents. This consistory paragraph does not correspond to claim 1, which is much narrower.
- At [0016]-[0019] there are consistory paragraphs identifying further aspects of the invention: a method of controlling the incorporation of a nucleotide molecule complementary to the nucleotide in a target single-stranded polynucleotide in a synthesis or sequencing reaction; a method for determining the sequence of a target single-stranded polynucleotide; and a kit comprising a plurality of different individual nucleotides of the invention and packaging.
- At [0020]-[0024] details are given of the nucleotide itself and of the proposal to use a linker to link the base to a detectable label. Further details of suitable labels and linkers are given in a passage from [0063] to [0090]. The preferred detectable labels are fluorophores, which can be detected by fluorescence.
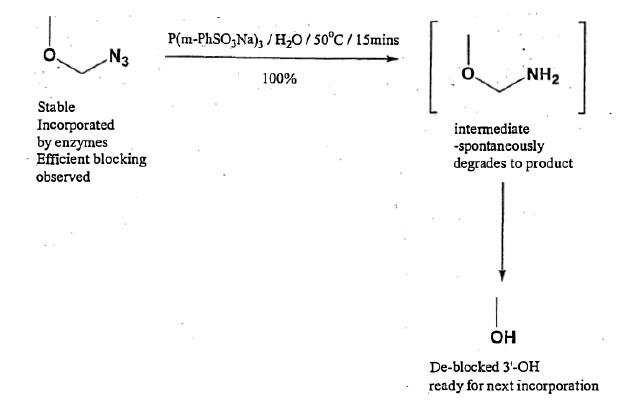
- In [0050] the specification states that one example of -O-Z is where Z is azidomethyl. Removal of azido groups is discussed in [0051].
- From [0102] to [0147] the specification describes a series of examples of the invention in which azidomethyl is the blocking group. This passage begins with the statement at [0103]:
“Nucleotides bearing this blocking group at the 3' position have been synthesised, shown to be successfully incorporated by DNA polymerases, block efficiently and may be subsequently removed under neutral, aqueous conditions using water soluble phosphines or thiols allowing further extension.”
- At [0104] to [0139] the synthesis of modified nucleotides with a 3'-OH azidomethyl blocking group, linked to a fluorescent dye via a linker moiety, is described. Syntheses of modified versions of all four of G, C, A and T with the triphosphate and a 3'-O-azidomethyl group are detailed. Compounds 6, 18, 24, and 32 are used in experiments. These are dTTP, dCTP, dGTP and dATP respectively.
- Experiments showing blocking, incorporation and deblocking using the modified nucleotides are described in [0140]-[0147] with the results shown in Figures 5 and 6. The specification states in [0147] that two cycles of incorporation with compounds 18 (C), 24 (G) and 32 (A) and six cycles of incorporation with compound 6 (T) can be seen.
- The experiments used radiolabelled “hairpin” primers, attached to beads, into which the modified nucleotides were incorporated. There were three stages: (a) incorporation of the modified nucleotide; (b) a chase by native unmodified nucleotides to check that incorporation of the modified nucleotide and thereby blocking of further incorporation had occurred; and (c) deblocking of the modified nucleotide to remove the blocking group and fluorescent label. This is shown in a diagram produced by Prof Leadlay:
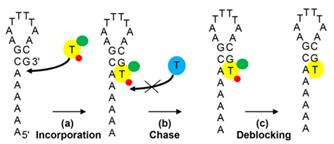
- At each stage, beads were removed from the reaction and the DNA was released from the beads onto a gel to allow analysis of the reaction products. The position of the bands on the gel corresponds to the size of the DNA: larger molecules move more slowly and are therefore visualised higher up the gel than smaller ones. The radiolabel on the hairpin permits the bands to be visualised.
- Fig. 5 shows the results for compounds 24, 18 and 32 (G, C and A). Prof Leadlay provided an annotated version of the figure which the judge accepted as showing how it would be understood by the skilled team:

- The hairpin band (to the left of each set) shows the position of the hairpin primer (prior to any incorporation) on the gel. Moving from left to right, the bands show the position of the DNA following the first cycle of incorporation; chase and deblocking phases and then the second cycle. The higher position of the band indicates a larger DNA molecule and hence shows that incorporation has occurred. Similarly, the lower position of the band indicates a smaller DNA molecule, and hence shows that de-blocking (and removal of the fluorescent label) has occurred.
- The judge found that the first cycle for compounds 18 and 32 shows complete incorporation and de-blocking of the modified nucleotides. The first cycle for compound 24 shows that there was also incorporation and de-blocking, but it was not complete as there is a faint band at the same position as the hairpin primer band in lane 1, indicating that some hairpin primers remained into which no nucleotide had been incorporated.
- Fig. 6 shows the results for compound 6 (T). Again, Prof Leadlay provided an annotated version which the judge accepted:
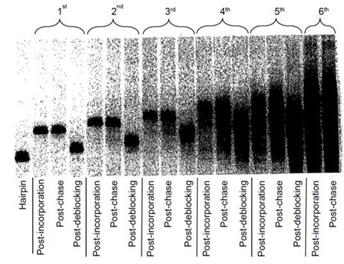
- As with Fig. 5, the lanes from left to right represent: hairpin primer followed by the first and then subsequent rounds of incorporation, chase and de-blocking. The results for the first three cycles show clearly that a modified nucleotide incorporates into the polynucleotide at each cycle and is then successfully de-blocked.
- It can be seen that, in general, in both sets of gels the bands widen as one goes from left to right. Little can be made out relating to the second cycles in Fig. 5 and the fourth, fifth and sixth cycles in Fig. 6.
- Having considered the evidence of the experts concerning the interpretation of the gels, the judge found at [148] that:
“… the skilled person would not simply disbelieve or reject what is said in paragraph [0147]. Rather they would accept that the second cycle for C, and A (less so for G) and the fourth and later cycles for T were likely to have taken place but may not have been fully efficient, and may have involved side reactions.”
- The judge went on at [150] to accept Prof Leadlay’s evidence which the judge summarised as follows:
“The data shows that modified nucleotides with a 3' azidomethyl blocking group may be used for controlled, one at a time, incorporation of nucleotides into a polynucleotide. The 3' azidomethyl modified nucleotides were incorporated by the polymerase, resulting in chain termination. The blocking group and fluorescent label are capable of being removed using a water-soluble phosphine to regenerate the 3' hydroxyl, allowing further rounds of incorporation of 3' blocked nucleotides in a stepwise manner.”
- The judge concluded his review of the specification by finding that:
“151. … the skilled [team] taking the patent as a whole including these figures 5 and 6 would conclude that while further optimisation of the conditions was likely to be required, they would expect it to amount to routine work. They would regard the data as showing that the incorporation and deblocking steps were sufficiently efficient to be a promising repeatable technique. The worst one was for compound 24 (G) but it was not so bad that the skilled [team] would think it would not work. Furthermore …. it would in fact be routine work for the skilled [team] to carry out.
152. Overall, the skilled [team] reading the patent as a whole and taking into account the experimental results, would accept as plausible the proposition that a nucleotide with a 3' azidomethyl blocking group satisfied the objectives set out by paragraphs [0004] and [0005].”
The claims
- It is only necessary for the purposes of the appeal to refer to claims 1 and 6 of 289. Claim 1 is as follows:
“A modified nucleotide triphosphate molecule comprising a purine or pyrimidine base and a deoxyribose sugar moiety having a 3'-azidomethyl group.”
- Claim 6 as amended (claim 10 as granted) is as follows:
“A method for determining the sequence of a target single-stranded polynucleotide, comprising monitoring the sequential incorporation of complementary nucleotides, wherein at least one incorporation is of a nucleotide comprising a purine or pyrimidine base and a deoxyribose sugar moiety having a 3’-azidomethyl group where the nucleotide has a base that is linked to a detectable label via a cleavable linker and wherein the identity of the nucleotide is determined by detecting the label linked to the base and the blocking group and label are removed prior to introduction of the next complementary nucleotide.”
- As the judge noted at [164], claim 12 (as amended, claim 17 as granted) of 578, to which claim 6 of 289 is equivalent, requires “at least one” incorporation. He went on at [292]-[301] to dismiss a case of insufficiency advanced by MGI based on the fact that the claim only requires one incorporation and has no upper limit to the number of incorporations. There is no appeal by MGI on the issue of insufficiency, but as will appear the judge rejected an argument that a conclusion that there was no insufficiency would be inconsistent with a finding of non-obviousness and MGI rely upon what he said in that context.
Zavgorodny
- Zavgorodny describes a method for synthesising certain substituted nucleosides. A nucleoside differs from a nucleotide in that it lacks the 5' phosphate groups. In other words, a nucleoside is just the base and the deoxyribose (or ribose). Nucleosides had a number of applications at all material times, including as antiviral and anticancer agents. The disclosure is summarised in the abstract as follows:
“Treatment of appropriately protected nucleosides with a mixture of acetic acid, acetic anhydride and dialkylsulfoxide was shown to give O-(1-alkylthioalkylated) nucleosides that were oxidised to the corresponding sulfoxides and sulfones, or converted via O-halogenomethyl derivatives to various O-substituted nucleosides.”
- Zavgorodny’s syntheses are summarised in the single figure in the paper, which is reproduced below.

- The synthetic scheme involves starting with a 5' blocked nucleoside and generating a 3'-O-methylthiomethyl nucleoside. That product is compound 1 in the scheme (second from the left at the top). The 5' hydroxyl position is blocked by a benzyl group (“Bz”). Zavgorodny treats the specific methylthiomethyl group used in the experiments as representative of the wider class of alkylthioalkyl groups.
- From the 3'–O-methylthiomethyl compound 1 various further routes are taken to generate a selection of nucleosides O-substituted at the 2', 3' and 5' positions. One of the methods used is to block the 3'-hydroxyl with an azidomethyl group. That is depicted in Zavgorodny’s figure as compound 5 (in which the 5' end is no longer blocked) where “X” is N3. Compound 5 is a deoxyribonucleoside.
- The last paragraph of Zavgorodny states (footnotes omitted):
“The compounds discussed above are useful specifically blocked synthons. For example, alkylthioalkyl groups can be removed with methyl iodide, mercury(II) and silver(I) salts, tritylium tetrafluoroborate, or bromine/water treatment. O-Methoxymethyl substituted nucleosides may be deblocked according to Nishino, and acetoxymethyl and 2-cyanoethoxymethyl groups undergo elimination under alkaline conditions. Azidomethyl group is of special interest, since it can be removed under very specific and mild conditions, viz. with triphenylphosphine in aqueous pyridine at 20 ºC.”
- The judge held at [189] that the first sentence reflected the focus of Zavgorodny as a paper about synthetic chemistry:
“… A ‘synthon’ is a unit to be used in further syntheses. This sentence emphasises that what Zavgorodny is providing are chemical structures which, from Zavgorodny’s point of view, are themselves going to be used to synthesise other things. In other words, … they are synthetic intermediates. …”
- The judge went on to consider the significance of the final sentence:
“190. … It draws attention to the azidomethyl group, saying it is of special interest and can be removed under very specific and mild conditions. However it is important to appreciate that the reference to ‘special interest’ is not an assertion about sequencing by synthesis reactions. Zavgorodny is not suggesting that this group is of ‘special interest’ in that context. The skilled [team] would understand that what Zavgorodny is talking about is in the context of its use as a synthon, a chemical intermediate. Protecting groups are a routine tool in synthetic chemistry. It would be an exercise of hindsight to read that as a disclosure concerned with sequencing by synthesis or reversible chain terminators.
192. Finally a point arises on what ‘triphenylphosphine in aqueous pyridine at 20 ºC’ means. It refers to using triphenylphosphine in what a skilled person would regard as an organic solvent: aqueous pyridine. It is common ground that pyridine denatures DNA.”
The difference between the claims and Zavgorodny
- The judge identified the difference between the claims and Zavgorodny at [193]:
“In a way the difference between Zavgorodny ... and claim 1 of 578 [as amended, equivalent to claim 1 of 289] is quite small. Claim 1 claims a nucleotide (i.e. with the 5' phosphates) with an azidomethyl group at the 3' oxygen whereas Zavgorodny … discloses a nucleoside with such a group at the 3' oxygen. However in order to render claim 1 invalid the skilled [team] has to make the claimed molecule and for that to happen the skilled [team] has to have a reason to do so. As a result the inventive step(s) of all the relevant claims of the modified nucleotide patents stand or fall together. If performing sequencing by synthesis using a nucleotide with an azidomethyl blocked 3' oxygen as a reversible chain terminator is obvious over Zavgorodny …, then claim 1 also lacks inventive step because it would be obvious to make the relevant compound. If that exercise was not obvious then none of the claims, including claim 1 of 578, are obvious for the converse reason. Another way of approaching the same question would be to ask whether it was obvious to the skilled [team] given Zavgorodny …, with a reasonable prospect of success, to try out a sequencing by synthesis test using a nucleotide with an azidomethyl blocked 3' oxygen as a reversible chain terminator.”
Obviousness over Zavgorodny
- The law. The judge briefly outlined the applicable principles at [179]-[182]. He set out the structured approach to the assessment of obviousness re-stated by Jacob LJ in Pozzoli SpA v BDMO SA [2007] EWCA Civ 588, [2007] FSR 37 at [23] and referred to Lord Hodge’s review of the law in Actavis Group PTC EHF v ICOS Corp [2019] UKSC 15, [2019] RPC 9. He also cited a passage from the judgment of Laddie J in Inhale Therapeutic Systems Inc v Quadrant Healthcare plc [2002] RPC 21 at [47] which it is worth setting out slightly more fully:
“… A fiction in patent law is that the notional uninventive skilled man in the art is deemed to have read and assimilated any piece of prior art pleaded by the party attacking the patent claim. If the invention is obvious to that person in the light of a particular piece of prior art, the claim in invalid. It is no answer to say that in real life the prior art would never have come to the attention of a worker in the field, for example because it was tucked away on the top shelf of a public library or because it was in a language which nobody in the art knew. The notional skilled person is assumed to have read and understood the contents of the prior art. However that does not mean that all prior art will be considered equally interesting. The notional skilled person is assumed to be interested in the field of technology covered by the patent in suit, but he is not assumed to know or suspect in advance of reading it that any particular piece of prior art has the answer to a problem he faces or is relevant to it. He comes to the prior art without any preconceptions and, in particular, without any expectation that it offers him a solution to any problem he has in mind. Some pieces of prior art will be much more interesting than others. A document directed at solving the particular problem at issue will be seized upon by the skilled addressee. Its very contents may suggest that it is a worthwhile starting point for further development. But the same may not be the case where a document comes, say, from a distant and unrelated field. …”
- The judge’s reasoning. The judge began by observing at [195]:
“As I have already mentioned, Prof Marx’s evidence that the invention was obvious was based on a premise that the skilled person would look at Zavgorodny with the specific aim in mind of finding a blocking group they might be able to use in a reversible chain terminator sequencing process, and the cross-examination of Prof Leadlay was on the same premise. However I have rejected this premise. It is tempting therefore simply to stop at this point and find the claim is not obvious.”
As he explained at [197], however, it was necessary for him to resist that temptation because the court’s task was to consider the evidence as a whole and come to a conclusion.
- The judge proceeded to reject MGI’s case that the claims were obvious in the light of Zavgorodny for four separate reasons. His first reason was as follows:
“198. The skilled [team] is a team working on research into sequencing by synthesis. They are aware of the idea of using reversible chain terminators but as far as they were concerned the idea had not succeeded. They knew that to make it work they would need to come up with a system in which one could repeatably incorporate a nucleotide linked to a specific label, one at a time, in a reversible way. They did not have any specific problem or problems in mind which had to be solved as a key to unlock the ability to take the method forward. It may well have been that the technique simply could not be made to work.
199. As a matter of principle, as with any item of prior art, the skilled [team] is deemed to read Zavgorodny with interest. They would see that it was a paper concerned with chemical intermediates (synthons). They would see that one such intermediate was a nucleoside in which the 3' OH had been blocked with azidomethyl. They would see the reference to removal using mild and specific conditions and that Zavgorodny regarded the azidomethyl group as of special interest. In my judgment the most likely thing such a skilled team would think having read Zavgorodny is that this paper on synthetic chemistry had nothing to do with their focus on sequencing by synthesis. To the skilled [team] the concept of protecting groups in synthetic chemistry is commonplace. At most Zavgorodny would be seen as something to add to the organic chemist team member’s general toolbox concerning chemical synthesis …. There is simply nothing, absent hindsight, to suggest that what is disclosed here has an application in relation to sequencing by synthesis using reversible chain terminators. They would read it with interest and having done so, put it down and move on.”
- The judge’s second reason was as follows:
“201. Even if the skilled [team] saw an analogy between the blocking 3' end in Zavgorodny and the idea of blocking the 3' end of a nucleotide in sequencing by synthesis, it would not make the invention obvious. The skilled [team] did not think they needed a new group to try as a reversible chain terminator. The skilled [team] knew that there were numerous possible candidate groups and would be aware that there was a textbook in which to find such things if they had wanted help with thinking of some to try (Greene & Wuts).
203. The skilled [team] would understand the reference to specific and mild conditions as a reference to the circumstances of Zavgorodny itself rather than a suggestion about the properties of this group in conditions required for DNA synthesis. On the other hand as a matter of the common general knowledge of the organic chemist member of the team, if they did get this far, they would think that they would be able to select conditions using their own skill which would be likely to remove such an azide group from a nucleotide without being likely to cause difficulties for DNA. …”
- The judge gave his third reason at [204]:
“Even if the skilled [team] got as far as considering whether to try out an azidomethyl group as a 3' blocking group on a nucleotide in a test of a single cycle, they would, as Prof Leadlay explained, have no basis for thinking that such a blocked nucleotide would be incorporated into an oligonucleotide by DNA polymerase. It might or it might not.”
- The judge’s fourth reason was as follows:
“209. …. While the skilled [team] would think they could come up with conditions in which to remove an azidomethyl group without damaging DNA, that is not the only issue. To be useful in sequencing by synthesis the removal has to have a reasonable yield and reasonable speed.
210. In my judgment the position is simply that the skilled [team] has no basis from which to infer that there was a reasonable prospect of getting a reasonable yield and speed.”
- By way of a cross-check, the judge concluded his assessment at [213] with a summary of the position with respect to each of the nine factors listed by Lord Hodge in Actavis v ICOS at [65]-[73], including the following:
“i) Obvious to try - I reject MGI’s submission that there was a sufficient likelihood of success to warrant trying incorporation of the azidomethyl with a range of standard polymerases. There would be no such expectation.
ii) Routine work - The work actually involved in testing a range of polymerases or testing deprotection is not difficult to do.
…
vi) Motive - I reject MGI’s submission that this points in favour of obviousness. On the contrary it points against for the reasons already addressed.
vii) Unexpected result - A 3'-O-azidomethyl blocking group has the useful features promised by the patent in paragraph [0004] and [0005]. That was not predictable from the prior art.
…”
- As noted above, the judge returned to the question of obviousness when considering insufficiency. In that context he said this:
“297. MGI is right that this conclusion highlights that all that needed to be obvious to invalidate claim 12 was to sequence a single … nucleotide using the 3' azidomethyl blocked nucleotide (with linker etc.). … The problem for MGI is that this dimension to the arguments on inventive step does not help MGI on the facts. The conclusion rejecting insufficiency is not inconsistent with the finding of non-obviousness.
298. What MGI is really trying to say is that because (say) Metzker 1994 did show the sequencing of one nucleotide, it follows that there is no real technical advance in this case because the claim only requires the sequencing of one nucleotide. Or similarly, that it is not open to hold (as I have) that the common general knowledge of the skilled [team] was that the technique of sequencing by synthesis using reversible chain terminators may well be something which could not be made to work, among other reasons because it was not reliably repeatable, because all that one needs to do to make the claimed invention work is sequence one nucleotide, and that was feasible based on Metzker. Neither point is right. There is a technical advance for the reasons already referred to. Essentially, the azidomethyl blocking group does meet the stringent requirements referred to in the patent. As for the common general knowledge, the state of the common general knowledge is a matter of fact unaffected by the scope of the claim. The fact that armed with the patent the skilled [team] only has to sequence one nucleotide to satisfy claim 12 does not mean the common general knowledge changes. Nor does it, in fact, alter the reasons why an azidomethyl blocking group was not obvious over Zavgorodny.”
- The appeal. Obviousness involves a multi-factorial evaluation and therefore this Court is not justified in intervening in the absence of an error of law or principle on the part of the judge: see Actavis v ICOS at [78]-[81]. This is particularly so given that the decision was one of an experienced specialist patents judge after hearing extensive oral evidence from experts in the field.
- MGI contend that the Modified Nucleotide Patents cannot be both non-obvious over Zavgorodny and entitled to priority from P2. At trial MGI’s primary case was that the Patents were obvious, and, as explained in more detail below, priority was run as a squeeze. Given the judge’s conclusion on obviousness, MGI’s primary case on the appeal is that the judge’s reasoning on priority is inconsistent with his reasoning on obviousness and that he ought to have concluded that the Patents were not entitled to priority. In the alternative, MGI argue that, if the judge was right on priority, then he was wrong on obviousness. Given the way the case was put at trial, I consider that the correct course is first to consider whether the judge made any error in his assessment of obviousness. I shall address the allegation of inconsistency when I come to consider priority.
- MGI do not suggest that the judge misdirected himself as to the law. Nor do they challenge his findings of fact as to the common general knowledge, his analysis of the disclosure of Zavgorodny or his identification of the difference between the claims and Zavgorodny. MGI argue that the judge should have concluded that the skilled team would have (i) appreciated the relevance of Zavgorodny to their research, (ii) been interested in the 3'-OH azidomethyl blocking group and (iii) tried incorporating a 3'-O-azidomethyl nucleotide into a chain and deblocking it in what the judge accepted were routine tests, and that the judge can only have reached the opposite conclusions on the basis of hindsight. The problem with this argument is that it merely amounts to a challenge to the judge’s evaluation of these points without identifying any error of principle on his part. Nevertheless, I will consider the main submissions made in support of it.
- First, counsel for MGI took issue with the judge’s characterisation of his cross-examination of Prof Leadlay at [121] and [195] as being essentially on the premise that the skilled person came to Zavgorodny interested in taking forward sequencing by synthesis with a new reversible chain terminator. Counsel was able to demonstrate by reference to the transcript that he had not explicitly asked any questions on that basis. The judge clearly took the view, however, that that was the underlying premise of the line of questioning. Given that the judge had the advantage of witnessing the cross-examination, it is not possible for this Court to conclude that he was not entitled to take that view. I would go further, however. Having read the transcript, I agree with the judge’s view that the cross-examination was based on the unexpressed assumption that what the skilled team would be looking for was a new reversible chain terminator i.e. a new blocking group. In any event, this submission goes nowhere given that MGI do not challenge the judge’s findings as to common general knowledge.
- Secondly, counsel for MGI submitted that the judge’s first reason for concluding that the claims were not obvious over Zavgorodny, namely that the skilled team would not have considered that Zavgorodny was relevant to their research, was inconsistent with his findings as to common general knowledge. I do not accept this. The judge made a clear finding at [126] that the skilled team was aware of the concept of using reversible chain termination and knew that this had not yet succeeded in practice, but did not have any specific problem in mind. The judge repeated this finding at [198], and it formed the foundation for his conclusion in [199] that it was only with hindsight that Zavgorodny could be seen to be relevant to research into sequencing by synthesis. Counsel for MGI argued that, not having any specific problem in mind, the skilled team would be receptive to the advantageous new blocking group disclosed by Zavgorodny. This simply does not follow, however. The skilled team would only be interested in a new blocking group if they thought that problems with existing blocking groups were the reason for the failure of previous groups to make the idea of reversible chain termination work in practice, but that was not the case.
- Thirdly, counsel for MGI submitted that the judge wrongly treated Zavgorodny in effect as being from a different technical field, particularly given that the judge accepted at [200] that it did not matter than Zavgorodny was concerned with nucleosides rather than nucleotides. Again, I do not accept this. Zavgorodny is not concerned with sequencing by synthesis, it is concerned with synthetic methods. Thus it is not from the same technical field as the claimed invention. While Zavgorodny is from a neighbouring technical field, the judge was entirely justified in characterising it as simply an addition to the organic chemist’s toolbox.
- Fourthly, counsel for MGI submitted that Prof Leadlay had accepted that, on the assumption that (as the judge found) the skilled team knows about sequencing by synthesis using reversible chain terminators, Zavgorodny’s statement that an azidomethyl blocking group could be removed “under very specific and mild conditions” would be of interest to the skilled team, and therefore the judge’s first and second reasons were both wrong. This point overlaps with the first point: as discussed, the judge interpreted the premise of the cross-examination differently and I agree with him. In any event, I do not accept counsel’s characterisation of Prof Leadlay’s evidence. While he did not say that, having read Zavgorodny, the skilled team would, as the judge put it, “put it down and move on”, his evidence was that the skilled team would note that Zavgorodny refers to “a number of other products which are known, established, well-characterised hydroxyl blocking groups” which would be likely to impress the team as much as azidomethyl. He went on to say that he did not think that the skilled team would be “motivated to use [azidomethyl] for sequencing, because there is simply not enough information to allow [them] to see that this matches the stringent requirements for such a blocking group” in sequencing. Later on, he said that Zavgorodny taught how to make six or seven blocking groups, most of which were “more attractive, on the basis of the conditions for their removal being better understood, and they would be taken forward, in my view, in preference to azidomethyl”. He went on to say that the team would be motivated to look again at “groups that had been established as having some of the features of the desired blocking group, and that includes O-nitrobenzyl”. The cross-examination culminated in the following exchange:
“Q. But [Zavgorodny says azidomethyl] is of special interest because it can be removed under very specific and mild conditions, and that would be particularly attractive to someone who was thinking of SBS using reversible chain terminators?
A. I think that that is coloured by hindsight. I think that the skilled person would rather look closely at the fact that the conditions in Zavgorodny simply do not tell you anything about the technical details of how the experiments should be done. You are in the dark. I think if you were forced to take azidomethyl on, if you took it on, it would be entirely speculative.”
In short, Prof Leadlay’s evidence fully supports the judge’s conclusion that the skilled team would simply regard Zavgorodny as adding to the repertoire of potential blocking groups if they were minded to try a new blocking group as a way of moving forward with sequencing by synthesis.
- Fifthly, counsel for MGI submitted that the judge’s third and fourth reasons, both of which go to the question of whether the skilled team would have had a reasonable expectation of success if they were to try using an azidomethyl blocking group in sequencing by synthesis, were wrong. The third reason was said to be inconsistent with the judge’s findings as to common general knowledge and with his findings on priority, while the fourth reason was said to be inconsistent with the claims and the disclosure of the Modified Nucleotide Patents. MGI accept that the skilled team would need to do experiments to find out whether a 3'-O-azidomethyl blocked nucleotide was incorporated by polymerase and could be deblocked without denaturing DNA, but say that such experiments were routine and the skilled team could select suitable polymerases, linkers, cleavage conditions and detectable labels without difficulty.
- The first of these points is a bad one. Nothing in [204] is inconsistent with the judge’s findings as to common general knowledge. The passages in the judgment relied upon in support of the submission are [151] (quoted in paragraph 42 above) and [285] (“there will be polymerases that do not work but the skilled person, given the information in the patent and the common general knowledge, will be able to select suitable polymerases without difficulty”). Both are concerned with the ability of the skilled team to put the teaching of the specification of the Modified Nucleotide Patents into practice. There is no inconsistency between saying a skilled team who had not read the Patents would not know whether a 3'-O-azidomethyl blocked nucleotide would be incorporated by DNA polymerase and saying that a skilled team who had read the Patents, which show that it is incorporated, would be able to optimise the method, and find other suitable polymerases, through routine work.
- The second point is more conveniently addressed in the context of priority, and I will return to it below.
- As for the third point, counsel for MGI pointed out the claims do not require a reasonable yield and reasonable speed, all they require is one cycle of incorporation and de-blocking. The judge was well aware of this, however, as can be seen from what he said at [191] and [297]-[298]. This does not detract from his conclusion at [204] that the skilled team would have no reason to think that there was a reasonable prospect of getting a reasonable yield and speed, and therefore a 3'-O-azidomethyl group was not obvious to try.
- Finally, counsel for MGI argued that the judge had failed correctly to characterise the problem solved by the invention and the narrow technical contribution made by it. Again, this argument is more conveniently addressed in the context of priority.
- I therefore conclude that the judge did not make any error in finding that the Modified Nucleotide Patents were not obvious in the light of Zavgorodny.
P2
- P2 is a considerably shorter document than 289. The general disclosure is similar to that of 289, and in particular it clearly discloses the use of an azidomethyl 3'-OH blocking group in sequencing by synthesis. The text contained in [0004] of 289 is to be found at page 21 line 36 to page 2 line 22 and the text contained in [0005] is to be found at page 9 line 28 to page 10 line 14 apart from the first five words (and those words are arguably implicit from the context provided by the preceding paragraph). P2 contains none of the experimental detail to be found in 289 at [104]-[147], however. Rather, it contains three examples which are described in less detail.
- Example 1 is described on page 23 as follows:
“1. 3' -OH protected with an azidomethyl group as a protected form of a hemiaminal:

Nucleotides bearing this blocking group at the 3' position have been shown to be successfully incorporated by a number of different polymerases, block efficiently and may be subsequently removed under neutral, aqueous conditions using water soluble phosphines or thiols allowing further extension:
 ”
”
- It can be seen that the paragraph above the synthetic scheme is identical to [103] of 289 save that the latter contains the additional word “synthesised” in the second line.
- It is not necessary to set out Examples 2 and 3 in detail, but it should be noted that Example 2 describes a different 3'-OH blocking group and states:
“Nucleotides bearing this blocking group have similar properties to the above example, though incorporate less rapidly.”
- The judge accepted at [237] that there was textual support in P2 for all the relevant claims. This does not appear to have been in issue before him, and it was common ground in this Court.
- The judge went on:
“238. MGI contends that the second priority document contains no data to support the claimed utility. Even in its own terms that is not the whole story. Example 1 on page 23 provides ….
239. This assertion does not have any graphs or gels associated with it, but it is a statement that experiments have been done and that they were successful in various specific ways which are relevant to success from the point of view of the skilled person. Prof Leadlay’s evidence, which I accept, was that this document:
‘clearly discloses … the utility of 3'-O-azidomethyl blocked nucleotides as reversible chain terminators in a sequencing by synthesis method. For example, [the document] discloses on page 23 that such modified nucleotides have been shown to be successfully incorporated by a number of different polymerases, block efficiently, and may subsequently be completely removed with 100% yield under neutral, aqueous conditions using water soluble phosphines or thiols, allowing further extension of the oligonucleotide chain.’”
Priority
- The law. In order for a claimed invention to be entitled to priority from an earlier application, it must, in the words of section 5(2)(a) of the Patents Act 1977, be “supported by matter disclosed” in that earlier application. Article 87(1) of the European Patent Convention expresses the requirement as being that priority can only be accorded in respect of “the same invention” as one in the earlier application. Section 5 is one of the sections which is declared to be intended to have the same effect as the corresponding provision of the EPC: see section 130(7). Both section 5 and Article 87 EPC give effect to Article 4 of the Paris Convention for the Protection of Intellectual Property.
- In case G 2/98 [2001] OJ EPO 413 the Enlarged Board of Appeal of the European Patent Office expressed the test for priority as follows:
“The requirement for claiming priority of ‘the same invention’, referred to in Article 87(1) EPC, means that priority of a previous application in respect of a claim in a European patent application in accordance with Article 88 EPC is to be acknowledged only if the skilled person can derive the subject-matter of the claim directly and unambiguously, using common general knowledge, from the previous application as a whole.’”
- Jacob LJ explained this test in Unilin Beheer NV v Berry Floor NV [2004] EWCA Civ 1021, [2005] FSR 6 at [48] as follows:
“The approach is not formulaic: priority is a question about technical disclosure, explicit or implicit. Is there enough in the priority document to give the skilled man essentially the same information as forms the subject-matter of the claim and enables him to work the invention in accordance with that claim?”
- It can be seen that Jacob LJ’s formulation has two aspects: disclosure (does the priority document give the skilled person essentially the same information as the claimed invention?) and enablement (does the priority document enable the skilled person to perform the claimed invention?)
- So far as the first aspect is concerned, Kitchin J (as he then was) added in Abbott Laboratories Ltd v Evysio Medical Devices plc [2008] EWHC 800 (Pat), [2008] RPC 23 at [228]:
“So the important thing is not the consistory clause or the claims of the priority document but whether the disclosure as a whole is enabling and effectively gives the skilled person what is in the claim whose priority is in question. I would add that it must ‘give’ it directly and unambiguously. It is not sufficient that it may be an obvious development of what is disclosed.”
This statement has subsequently been endorsed by this Court: see MedImmune Ltd v Novartis Pharmaceuticals UK Ltd [2012] EWCA Civ 134, [2013] RPC 27 at [154] and Icescape Ltd v Ice-World International BV [2018] EWCA Civ 2219, [2019] FSR 5 at [41].
- Floyd LJ explained this further in HTC Corp v Gemalto SA [2014] EWCA Civ 1335, [2015] RPC 17 at [65]:
“The skilled person must be able to derive the subject matter of the claim directly and unambiguously from the disclosure of the priority document. Mr Tappin stressed that the question was one of what was disclosed to the skilled person, not what was made obvious to him by the priority document, for example in the light of his common general knowledge. I agree that … that is the correct approach. That does not mean, however, that the priority document should be read in a vacuum. The question of what a document discloses to a skilled person takes account of the knowledge and background of that person. A document may mean one thing to an equity lawyer and another to a computer engineer, because each has a different background. The document still only has one meaning because it is only the relevant skilled person's understanding which is relevant. What is not permissible is to go further than eliciting the explicit or implicit disclosure and take account of what a document might lead a skilled person to do or try, or what it might prompt him to think of.”
- So far as the second aspect is concerned, it is settled that the test of enablement is the same in the context of priority as in the context of sufficiency of disclosure: see Asahi Kasei Kogyo KK’s Application [1991] RPC 486 and Biogen Inc v Medeva plc [1997] RPC 1 at 46-49.
- In Hospira UK Ltd v Genentech Inc [2014] EWHC 1094 (Pat) Birss J held as follows:
“147. Genentech submitted that the requirement for plausibility which is part of the law of sufficiency was not relevant in the context of priority and referred to a EPO decision T903/05 Gemvax in which the Technical Board of Appeal rejected the suggestion that to be entitled to priority it was necessary for the priority document to contain data which made it plausible that the claimed invention worked (paragraph 11). Hospira did not agree and submitted that in a case like this one, plausibility was as much part of the test for priority as it was part of the test for sufficiency. The investigation of whether an invention is plausible is part of the requirement for enablement. Hospira argued that enablement was not challenged in Gemvax.
148. Although Hospira are right that enablement was not challenged in Gemvax, the Board regarded plausibility as a separate requirement distinct from enablement. Genentech is right that the Board of Appeal held that plausibility was not a requirement for priority in any event.
149. Although I am reluctant to do so I disagree with the statement in Gemvax. The requirement for priority is that the earlier application must be in respect of the same invention as the patent. The establishment of priority includes a requirement for an enabling disclosure (Biogen v Medeva [1997] RPC at 48–49). In order to make an enabling disclosure of an invention it must be possible to make a reasonable prediction that the invention will work (Regeneron [v Genentech [2013] EWCA Civ 93, [2013] RPC 28] paragraph 100). In the context of an invention which includes the achievement of a therapeutic effect as one of its features, absolute proof is not required but the patentee must show that the therapeutic effect is plausible (Regeneron paragraph 103). It seems to me that this logic applies just as much to priority as it does to sufficiency of disclosure (see also Biogen on the relationship between priority and sufficiency). The alternative would be a recipe for abuse. A patentee could file a speculative priority application and obtain an earlier priority date, thereby stealing a march on the competition. I find that in law the test for priority includes the requirement for plausibility in a case like this one.”
- The decision in Hospira v Genentech was affirmed on appeal [2015] EWCA Civ 57, but priority was not in issue on the appeal. Birss J followed his statement of the law in Merck Sharp & Dohme Ltd v Ono Pharmaceutical Co. Ltd [2015] EWHC 2973 (Pat), [2016] RPC 10 at [127], but neither side contended to the contrary. It was also followed by Henry Carr J in Hospira UK Ltd v Cubist Pharmaceuticals LLC [2016] EWHC 1285 (Pat), [2017] RPC 10 at [114(v)], but the plausibility of the priority documents was not in issue in that case.
- Illumina does not challenge the accuracy of Birss J’s statement that “the test for priority includes the requirement for plausibility in a case like [Hospira v Genentech]”, which was a medical use case, but it disputes that plausibility is always required for priority. I shall return to this point below.
- Illumina does not dispute that, if plausibility is required, the criterion for plausibility is that stated by Lord Sumption in Warner-Lambert Co LLC v Generics (UK) Ltd [2018] UKSC 56, [2019] Bus LR 360 at [36]: “the specification must disclose some reason for supposing that the implied assertion of efficacy in the claim is true”. As Lord Sumption went on to explain at [37], a bare assertion is not enough. Definite proof is not required, however. Nor is experimental data necessarily required if the specification contains reasoning which would cause the skilled person to think that there was a reasonable prospect that the assertion would prove to be true.
- The final point on the law concerns the burden of proof. The legal burden lies upon the patentee to justify its claim to priority, but as Laddie J explained in Evans Medical Ltd’s Patent [1998] RPC 517 at 533 the evidential burden may shift to the party attacking the validity of the patent:
“Although the onus is on [the patentee], it would be strange if this had the result in most cases of requiring the patentee to carry out experiments simply to prove entitlement failing which the defendant could simply assert that early priority had not been proved. Although the onus is on the patentee, in a normal case the court will proceed on the basis that the technical results set out in the priority document can be achieved as asserted. It is enough if the patentee asserts that the instructions in the priority document work to produce the results claimed for them. A claim to priority is, implicit[l]y, such an assertion of enablement. If that is not effectively challenged then the patentee has done enough.
It follows that a defendant should do more than merely issue a challenge to priority. He must explain the manner in which he says there is failure to enable. This should be set out in his pleadings. He will have to support his assertions by evidence. The court should be able to proceed on the basis that the experimental data in the priority document is not only right but reasonably repeatable. Although the onus remains on the patentee, the evidential burden shifts to the defendant. In some cases he may not be able to discharge it without supporting experiments.”
- The judge’s reasoning. The judge recorded at [235] that the legal test for priority was not in dispute before him, and referred to Icescape v Ice-World. He went on to hold that the claims of the Modified Nucleotide Patents were entitled to priority from P2 for the following reasons:
“240. Based on this, it seems to me that the conclusion that the claims are entitled to priority must follow. The same invention is disclosed in both the priority document and the patent. The disclosure in the priority document supports the claims and is an enabling disclosure. It also provides plausible information which supports the idea that a sequencing by synthesis scheme based on the claimed 3' O-azidomethyl blocking group will work.
242. There is no squeeze relating to Zavgorodny and no inconsistency between this conclusion and the finding of non-obviousness.”
- Although he did not expressly say so, it appears from this reasoning that the judge proceeded on the basis that, as a matter of law, plausibility was required for priority.
- Illumina’s alternative case. In addition to supporting the judge’s decision on the facts, Illumina contend in the alternative that the judge was wrong to proceed on the basis that plausibility was required for priority as a matter of law. Although, for reasons that will appear, it is not necessary to decide whether this contention is correct, it is convenient to consider it before turning to MGI’s appeal because it illuminates the arguments on the appeal.
- Illumina did not file a respondent’s notice raising this contention. Counsel for Illumina submitted that no respondent’s notice was required because the judge had made no decision adverse to Illumina so far as priority was concerned. I disagree. A respondent who wishes to ask the appeal court to uphold the decision of the lower court for reasons different from or additional to those given by the lower court must file a respondent’s notice: CPR rule 52.13(2)(b). Given that (i) the point is a pure question of law, (ii) the argument was clearly raised in Illumina’s skeleton argument, (iii) MGI raised no objection when they received the skeleton argument and (iv) MGI were able fully to respond to the argument in oral submissions, however, I would if necessary give Illumina permission to file a respondent’s notice raising this point.
- In its skeleton argument Illumina contended that plausibility was only required for medical use claims, and that in other kinds of case all that was required for priority was disclosure and enablement. In the course of argument, however, counsel for Illumina retreated somewhat from this position. He accepted that plausibility was required not merely for medical use claims, but also in other kinds of case involving what he called “predictive claiming”, where the applicant was predicting that a class of products and/or methods would have utility across the breadth of the class.
- As I understood the revised submission, this would include a case such as Idenix Pharmaceutics Inc v Gilead Sciences Inc. In that case the claims were on their face pure compound claims defining a class of compounds by means of a Markush formula. It was common ground, however, that the validity of the claims should be assessed on the basis that they were to be interpreted as claims to compounds which had anti-Flaviviridae activity: see [2014] EWHC 3916 (Pat) at [306] and [2016] EWCA Civ 1089 at [5], [104]. It is clear that the reason why the patentee conceded that interpretation was that it realised that, otherwise, the claimed inventions would not be inventive because they made no technical contribution to the art, applying the reasoning in T 939/92 AGREVO/Triazoles [1996] EPOR 171 (although in the event they were held uninventive on that ground anyway, because it was not plausible that substantially all of the compounds had such activity).
- It may be noted that I stated at first instance in that case at [178] that:
“…. the question of plausibility must be tested by reference to the contents of the Application. If the claimed inventions are only plausible when considered by reference to the contents of the Patent, and not when considered by reference to the contents of the Application, then it must follow that the Patent is invalid for added matter.”
- When asked about this, counsel for Illumina’s initial response was to submit that this reasoning did not apply to priority. I do not understand why there should be any difference between priority and added matter in this respect, however, given that the legal tests are largely, if not entirely, the same: see Unwired Planet International Ltd v Huawei Technologies Co Ltd [2015] EWHC 3366 (Pat) at [106]-[107] (Birss J). So too is the underlying rationale of preventing patentees from obtaining an unwarranted advantage by subsequently adding subject-matter not disclosed in the priority document (priority) or in the application as filed (added matter): compare Birss J in Hospira v Genentech at [149] (priority) with Jacob LJ in Vector Corp v Glatt Air Techniques Ltd [2007] EWCA Civ 805, [2008] RPC 10 at [5]-[8] (added matter). This is another way in which the law gives effect to the fundamental principle articulated by the Technical Board of Appeal in T 409/91 EXXON/Fuel oils [1994] OJ EPO 653 at [3.4], cited with approval by Lord Hoffmann in Biogen v Medeva at 49 and by Lord Sumption in Warner-Lambert v Generics at [17], that “the patent monopoly should be justified by the actual technical contribution to the art”. As I understood his later submissions, counsel for Illumina accepted this in cases of “predictive claiming”.
- Counsel for Illumina submitted that the Modified Nucleotide Patents did not involve “predictive claiming”, and hence plausibility was not required. I disagree with the premise of this submission. Claim 1 of 289 is to a class of chemical compounds (the breadth of which was not explored either at trial or on the appeal), and claim 6 is to a method involving that class of compounds. As can be seen from the judge’s reasoning on obviousness, the judge proceeded on the basis that the invention did not lie in merely identifying this class of compounds, but in identifying their utility in sequencing by synthesis, and hence in the claimed method. That was why claim 1 and claim 6 stood or fell together so far as obviousness was concerned. Neither in P2 nor in the granted Patents did Illumina demonstrate that all, or substantially all, of the claimed compounds had the claimed utility. Rather, it relied upon a limited amount of evidence as supporting the proposition that all, or substantially all, of the claimed compounds had such utility.
- MGI’s appeal. In order to put the arguments on the appeal into context, it is important first to explain how MGI put their case on priority at first instance. As mentioned above, priority was run as a squeeze on obviousness: MGI argued that, if the claimed inventions were not obvious over Zavgorodny, then they were not entitled to priority over P2 because, MGI submitted, the skilled team would have been no better informed from a technical perspective after reading P2 than after reading Zavgorodny.
- MGI did not plead in their Grounds of Invalidity that P2 did not make it plausible that the claimed compounds would have the utility claimed for them in 289. Nor did MGI lead any evidence from Prof Marx to that effect or put any such case to Prof Leadlay in cross-examination.
- Still less did MGI plead or lead any evidence from Prof Marx or put to Prof Leadlay that the skilled team would think that Example 1 in P2 was a “prophetic” or “armchair” example, i.e. not a record of an experiment that had actually been performed, but rather a prediction of what would happen if it was carried out. The nearest MGI got to this was a statement in paragraph 12 of Prof Marx’s third report that the skilled person would view the statement that a 100% yield in the deblocking step was achieved “with a degree of scepticism as it is very rare for any reaction to proceed to 100% completion”. When this was put to Prof Leadlay, he disagreed on the ground that it is not uncommon for deblocking reactions to proceed to completion.
- Despite this, MGI submitted in its written closing submissions that “the disclosure [in Example 1] appears entirely theoretical and no evidence is provided that any worked example has been accomplished”.
- In these circumstances, I consider that the judge was not merely entitled, but correct, to find at [239] that Example 1 was to be taken at face value as “a statement that experiments have been done and that they were successful in various specific ways”. As Warby LJ pointed out during the course of argument, this finding is supported not merely by the wording of Example 1 and by the unchallenged evidence of Prof Leadlay quoted by the judge, but also by the statement in Example 2 quoted in paragraph 78 above.
- MGI nevertheless contend that the judge fell into error in concluding that P2 made it plausible that the claimed compounds had the utility claimed for them. In support of this contention counsel for MGI made two main submissions. Before turning to those I should explain that he accepted that the judge was entitled to interpret the claims in the manner I have discussed in paragraph 101 above.
- First, counsel for MGI pointed to the judge’s statement in [241] that it would not matter if Example 1 was a prophetic example because that would not render the disclosure any less plausible, and submitted that this demonstrated that the judge must have applied the wrong test. Counsel for Illumina accepted that this was a difficult passage to understand, and that if taken literally it would be contrary to common sense let alone legal principle. He submitted that the judge could not possibly have intended to contradict his own finding just two paragraphs beforehand at [239], and that what the judge must have meant was that any doubts the skilled team might have had would not have been sufficient to make them conclude that the experiment had not been performed. I accept this submission.
- Secondly, counsel for MGI submitted that the judge’s reasoning on priority was inconsistent with his reasoning with respect to obviousness in that the latter was predicated upon the Patents having made a major technical contribution to the art, whereas the former involved P2 having made a very minor technical contribution.
- The principal foundation for this submission was the judge’s findings at [148]-[152] (see paragraphs 40-42 above), and in particular his finding that the skilled team reading 289 as a whole and taking into account the experimental results in Figs. 5 and 6 would accept as plausible the proposition that a nucleotide with a 3'-O-azidomethyl blocking group satisfied the objectives set out in [0004] and [0005]. In addition, counsel for MGI relied upon the judge’s statements at [213(vii)] that a 3'-O-azidomethyl blocking group has the useful features promised by 289 in those paragraphs (see paragraph 60 above) and at [298] that the azidomethyl blocking group does meet the stringent requirements referred to in the patent (see paragraph 61 above). Thus, he submitted, the judge had assessed obviousness on the basis that the problem solved, and the technical contribution made, by the claimed inventions was the provision of a blocking group which plausibly met all of the “stringent requirements” in [0005] and thus enabled multiple cycles of incorporation and deblocking to be achieved as envisaged in [0004].
- By contrast, as counsel for MGI emphasised, P2 does not include Figs. 5 and 6. Even if the skilled team took Example 1 entirely at face value, he submitted, at best all this showed was that a single cycle of blocking, incorporation and deblocking could be achieved. That was no more than Metzker 1994 and Canard 1994 had achieved. Thus the problem solved, and the technical contribution made, by P2 could only be the provision of an alternative blocking group to the groups used by those workers. If that was the case, the claimed inventions were obvious. Given the conclusion that the claimed inventions were not obvious, however, the correct conclusion in relation to priority was that P2 did not make it plausible that a 3'-O-azidomethyl blocking group achieved the benefits promised in 289 at [0004]-[0005].
- Despite the skill with which these submissions were developed by counsel for MGI, I do not accept them. My reasons are as follows.
- First, I agree with the judge there is no squeeze between obviousness and priority in this case. Contrary to MGI’s case, the skilled team is better informed from a technical perspective after reading P2 than after reading Zavgorodny. Zavgorodny contains no hint that a 3'-O-azidomethyl blocking group should be used in sequencing by synthesis, whereas P2 not merely proposes this, but also states that nucleotides with this group have been successfully incorporated by a number of different polymerases, block efficiently and may be subsequently removed under neutral, aqueous conditions allowing further extension. Furthermore, it gives the experimental conditions for the unblocking step and states that it proceeds to completion. As discussed above, the skilled team would conclude from this that it was plausible that the claimed compounds had the utility claimed for them.
- Secondly, although MGI pleaded a case of AgrEvo obviousness against the Modified Nucleotide Patents, the case MGI ran at trial against the claims which are now in issue was a conventional case of obviousness over Zavgorodny. It is fair to say that, in that context, MGI argued that the technical contribution of the Patents was just the identification of an alternative blocking group. The judge did not accept that contention, but the reason why the judge rejected MGI’s case was not merely because of his assessment of the technical contribution made by the Patents, but more fundamentally because Zavgorodny did not point the skilled team towards the claimed inventions.
- Thirdly, even in closing submissions, MGI did not argue, or at least did not clearly argue, that the Modified Nucleotide Patents were not entitled to priority because P2 did not make it plausible that the claimed compounds would have the claimed utility. The nearest MGI got to this was to submit in its written closing submissions that Prof Leadlay did not “provide any comment as to whether the skilled person would view the disclosure of P2 as plausible”; but when pressed by the judge during oral closing submissions as to “what the ground of loss of priority is”, counsel for MGI’s answer was “lack of enablement”.
- Fourthly, even if that argument had been more clearly advanced, I consider that the judge would have been correct to reject it. Given Prof Leadlay’s unchallenged evidence quoted by the judge at [239], the judge was entitled to find, as he did at [240], that Example 1 made it plausible that a 3'-O-azidomethyl blocking group “will work”, that is to say, will satisfy the requirements described in the passages corresponding to [0004]-[0005] in 289 (see paragraph 75 above). It is true that, unlike 289, P2 does not include any experimental data showing more than one cycle of incorporation and deblocking, but it is clear from the judge’s findings as to common general knowledge and his remarks in the context of insufficiency that such data was not essential to render this plausible. Thus the data in the granted Patents simply made it more plausible.
Conclusion
- For the reasons given above I would dismiss MGI’s appeals with respect to the Modified Nucleotide Patents.
The 415 Patent
The skilled team
- The judge found at [424] that the 415 Patent was addressed to a skilled team consisting of three members, namely the two members of the team to whom the Modified Nucleotides Patent are addressed with the addition of a fluorescence chemist.
Common general knowledge
- The judge set out his findings as to the skilled team’s common general knowledge at [425]-[435]. For present purposes the following slightly abbreviated account will suffice.
- Various classes of fluorescent dye were known at the priority date, including xanthenes, which have a three-ring structure. Rhodamines are a sub-class of xanthenes in which amino groups are added to the xanthene core. Rhodamines were known to be useful for biological applications for a number of reasons. A well-known rhodamine was tetramethyl rhodamine:

The carboxy (COO-) group on the benzyl ring attached to the xanthene core is referred to as an ortho-carboxy group.
- In addition, the following matters were common general knowledge:
“430. …. the idea of functionalising a dye molecule in order to conjugate it to a biological molecule. Common functional groups are carboxyl groups and amino groups. They join together to form an amide bond. When the target biomolecule had one of them it was well known to put the other on the dye to create the amide. The ortho-carboxy group in rhodamines was used for this purpose. … Commercial dyes would often be available in a variety of different activated or functionalised forms to allow for conjugation to different biomolecules.
431. Linkers were a well known tool to use to connect a fluorophore to a biomolecule and help to prevent interaction between the two. A linker needs a functional group at each end to allow the two molecules to be joined. It should be stable under the relevant conditions and should not fluoresce. …
433. In terms of selecting fluorophores for labelling biological molecules, the skilled [team] would select a fluorophore with spectral properties suitable for their equipment and suitable to allow unambiguous detection. So if four fluorophores were to be used to sequence DNA, they would need to be distinguishable. The fluorophores should be bright and photostable and not interfere with the reactions taking place in sequencing by synthesis. …
435. However in the end this is an empirical field. The characteristics of individual moieties within a chemical structure are influenced by their environment. Molecules can be drawn out flat on a page but as the skilled [team] knows they are in fact three dimensional structures which can move. Parts which look remote on the page can interact with more distant parts because the whole molecule can adopt a shape in which that is true. Given a known fluorescent molecule and a new molecule derived from that known fluorescent molecule e.g. by adding a linker of some kind to it, and given the photochemical properties of both forms, the skilled [team] can rationalise whatever differences do or do not exist in the photochemical properties by reference to those structural changes. However that is not the same as being able to predict in advance with any degree of precision what the effect of a given change would be.”
The 415 Patent
- The specification begins by stating at [0001] that the invention provides novel rhodamine dye compounds, labelled conjugates comprising those dyes and methods for the use of these conjugates in sequencing by synthesis.
- At [0002]-[0006] the specification explains the background to the invention, noting that a need has developed for fluorescent dyes and their nucleic acid conjugates which satisfy various requirements for use in sequencing by synthesis.
- At [0008] the specification states that the claimed invention provides a compound of the formula shown below. As a result of an amendment to the claims, however, this no longer falls within the claims.
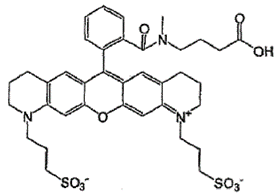
- At [0011] the specification states that also claimed is a compound of the formula shown below. Again, this no longer falls within the claims.

- At [0012] the specification states that a third aspect of the invention provides a nucleotide or nucleoside compound defined by the formula “N-L-Dye”, wherein N is a nucleotide, L is an optional linker moiety and Dye is a fluorescent compound according to the invention. Although the specification only describes L as a “moiety”, the skilled team would appreciate that L and Dye were moieties of the same molecule as well (rather than separate molecules).
- At [0013] the specification states that a fourth aspect of the invention includes methods of sequencing using the dye compounds of the invention.
- The specification goes on to disclose a number of dyes, including a rhodamine dye called Dye 2 at paragraph [0028]:
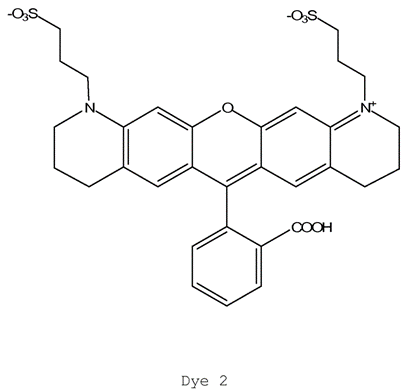
- The specification goes on:
“[0034] According to a second aspect of the invention there are provided dye compounds suitable for attachment to substrate moieties, particularly comprising linker groups to enable attachment to substrate moieties. Substrate moieties … may include nucleosides, nucleotides … More particularly the covalent attachment is by means of a linker group.
[0035] The dyes according to the invention may include a reactive linker group at one of the substituent positions for covalent attachment of the dye to another molecule. Reactive linking groups are moieties capable of forming a covalent bond. In a particular embodiment the linker may be a cleavable linker. … The cleavage site can be located at a position on the linker that ensures that part of the linker remains attached to the dye and/or substrate moiety after cleavage. … The use of a cleavable linker to attach the dye compound to a substrate moiety ensures that the label can, if required, be removed after detection, avoiding any interfering signal in downstream steps.”
- At [0037] the specification states:
“… the inventors have determined … that by altering, and in particular increasing, the length of the linker between a fluorescent dye (fluorophore) and the guanine base, by introducing a polyethylene glycol spacer group, it is possible to increase the fluorescence intensity compared to the same fluorophore attached to the guanine base through other linkages known in the art. The design of the linkers, and especially their increased length, also allows improvements in the brightness of fluorophores attached to the guanine bases of guanosine nucleotides when incorporated into polynucleotides such as DNA. Thus, when the dye is for use in any method of analysis which requires detection of a fluorescent dye label attached to a guanine-containing nucleotide, it is advantageous if the linker comprises a spacer group of formula -((CH2)2O)n- wherein n is an integer between 2 and 50 …”
- At [0044] the specification states that nucleosides or nucleotides labelled with dyes of the invention may have the formula shown below, in which Dye is a dye compound according to the invention, B is a nucleobase, such as uracil, thymine, cytosine, adenine and guanine, and L is an optional linker group.

- At [0075]-[0080] the specification discusses kits incorporating modified nucleosides and/or nucleotides labelled with dyes according to the invention. In this context it suggests a range of different dyes that may be used, according to various criteria. There is no suggestion of any interaction with the linker group.
- The specification describes a series of examples at [0083]-[0203], some of which are “reference” examples i.e. ones which do not fall within the granted claims.
- Example 1 first describes the synthesis of Dye 2, which is reported at [0091] to have an absorption maximum at 560 nm. It goes on to describe the attachment of a carboxyfunctional linker arm to the ortho-carboxy group of Dye 2 to form the compound shown below, which is reported at [0095] to have an absorption maximum at 570 nm:

- Example 4 describes the synthesis of an azide linker called LN3. This has the following structure:
The azide group roughly in the middle makes the linker cleavable.
- Example 5 describes the preparation of “‘Dye 2’-LN3” (building block language which is repeated in a number of other examples). This involves connecting together the derivatised dye (Dye 2 + carboxyfunctional linker arm), the azide linker (LN3) and a 3'-azidomethyl blocked nucleotide triphosphate. The nucleotide is thymidine. The resulting molecule is shown below:
- The specification then describes the synthesis of three other 3'-azidomethyl blocked nucleotide triphosphate molecules, each with a different fluorescent dye (Examples 6, 8 and 9). The result is a suite of four dye labelled conjugate molecules corresponding to nucleotides A, C, G and T, each of which is labelled with a different dye so that it can be separately identified by fluorescence.
- Example 9 relates to the base guanine. In this example the linker between the dye and the nucleotide includes 11 units of polyethylene glycol (PEG). This spaces the dye further away from the nucleotide for the reasons explained in [0037].
- Example 11 discloses the use of the four labelled modified nucleotides in a sequencing reaction of a DNA template of known sequence using fluorescent detection by a repeated cycle of incorporation and cleavage. The templates were scanned with four colours of light. The specification states at [0203]:
“The images were analysed to pick the brightest colour for each cluster, and this image intensity analysis was used to call the base for each cluster at each cycle. Images from each cycle were colocalised to obtain the sequence corresponding to each cluster. As the sequence of each cluster is known; and is the same for every cluster in the above experiment, the error rates (i.e. clusters not called as the correct sequence) can be analysed for each cycle of nucleotide incorporation. The error rates were less than 1% for the first 20 cycles of the experiment, meaning the known sequence of the mono-template was correctly identified.”
- As the judge found at [446]:
“This indicates that the [sequencing by synthesis] performed well and that the fluorescently labelled modified nucleotides produced a clear signal to permit reliable detection.”
The claim
- The only claim which it is necessary to consider is claim 1 as amended, claim 3 as granted:
“A nucleotide labelled with a compound according to the formula:
 ”
”
- As the judge explained at [448], this is a claim to a nucleotide labelled with the compound made from Dye 2 derivatised with a carboxyfunctional linker arm and linker LN3, i.e. a molecule of the formula N-L-Dye, to use the language of [0012]. One such molecule is the thymidine-based example shown in paragraph 136 above.
The prior art
- MGI contend that the 415 Patent is obvious over US Patent No. 4,900,686 (“Arnost”) published on 13 February 1990 and International Patent Application No. WO 2004/018493 (“Milton”) published on 4 March 2004. Arnost concerns fluorescent dye compounds and Milton concerns linkers. MGI say that the 415 Patent claims a mere collocation of a dye that is obvious over Arnost and a linker that is obvious over Milton.
- Milton. The judge considered the disclosure of Milton at [471]-[479]. Milton discloses the use of labelled nucleotides with cleavable detectable fluorescent labels in a sequencing by synthesis process. The essential idea disclosed is to conjugate a suitable fluorophore, via a suitable cleavable linker, to a suitably functionalised nucleotide.
- At page 18 lines 12-33 Milton explains that the method can be used with conventional detectable labels such as fluorophores. Dyes Cy3 and Cy5 are mentioned. The passage also says that other commercially available fluorescent labels include rhodamine.
- At page 21 lines 11-19 Milton explains that the linker can contain a spacer unit which distances the nucleotide base from the cleavage site in the linker or from the label attached to the linker. The length of the linker is unimportant as long as the label (i.e. the fluorophore) is held a sufficient distance from the nucleotide to avoid any interference between the nucleotide and an enzyme (i.e. a polymerase).
- At page 47 line 28 to page 48 Milton describes the synthesis of an azide linker which is the same as LN3 in the 415 Patent. The linker is attached to a Cy3 fluorescent dye at page 49 line 1 to page 50 line 9. The reaction is shown as follows:

- At page 50 line 11 to page 52 line 3 Milton describes the conjugation of this linked dye to a derivatised nucleotide to give a labelled nucleotide. The reaction is shown as follows:
- Milton explains that the linked dye and the dye-linker-nucleotide conjugate were each quantified by measuring their absorbance at 550 nm, indicating to the skilled team that these changes did not affect this aspect of the photochemical properties of Cy3.
- At page 52 line 5 to page 53 line 31 Milton describes two cycles of sequencing by synthesis using a thymidine form of the same labelled nucleotide. The results are shown in the form of gels using radiolabelled 32P. No results are presented based on using fluorescent detection. The gels show that the linker did not prevent incorporation of the nucleotide, although there is evidence in the gels that incorporation was not 100%. Incorporation at the second cycle is reasonably clear. The skilled team would see these results as supportive of the teaching of Milton that the linker should not interfere with the polymerase reaction.
- The judge held at [479] that:
“… there is no case that it would be obvious for that skilled person in those circumstances to alight on dye XVI of Arnost at all. The reference to rhodamines does not take the skilled person to Arnost on any view. … ”
- Arnost. The judge considered the disclosure of Arnost at [480]-[495]. Arnost discloses novel fluorescent conjugates comprising a rhodamine dye moiety and biologically active moiety for use in diagnostic assays. At column 3 line 43 a DNA probe is mentioned as one possible biologically active molecule, but there is no reference to sequencing by synthesis.
- Example III of Arnost discloses the synthesis of compound XVI shown below.
- As the judge explained at [489]:
“… molecule XVI of Arnost is Dye 2 of the 415 patent. Therefore Arnost discloses a molecule identical with the left hand side of the formula of claim 1 and teaches its use in making conjugates with biologically active molecules. Note however that molecule XVI stops at the ortho-carboxyl group on the benzyl group below the xanthene core. Arnost does not disclose the linker arm of claim 1 which goes from that carboxyl group to the middle amide of claim 1.”
- The judge went on to find as follows:
“490. … I am quite sure it was obvious for a skilled person at the priority date concerned with labelling biological molecules, given Arnost, to take molecule XVI forward as a candidate fluorophore. ….
493. Accordingly I find that given Arnost, an obvious molecule for the skilled person to make in the context of labelling biologically active molecules in a bioassay, is the left hand end of the formula of claim 1 of the 415 patent up to the middle amide. It is the 4 carbon option. I will call this molecule the 4 carbon linker form of dye XVI.
…
495. However, there is no case that it would be obvious for that skilled person in those circumstances to alight on the disclosure of Milton in these circumstances or onto a nucleotide conjugated to what is linker LN3 as shown (but not named that way) in Milton.”
- Summary. The judge summarised the position in the light of this prior art as follows:
“501. On the conclusions I have reached, it follows that claim 1 will be invalid if it is a collocation.
502. Claim 1 can be seen as a combination of two aspects:
i) the 4 carbon linker form of dye XVI; and
ii) a derivatised nucleotide plus linker LN3.
503. The place where they join is the middle amide bond. The findings so far mean that each of the two elements of claim 1, taken on its own, is obvious, but it would not be obvious to combine these two elements.”
Collocation
- The law. The principle that one cannot validly claim a mere collocation of two features each of which is old or obvious is one that has long been recognised in English patent law, and it has also been recognised in European patent law. It is a principle which needs to be treated with some care, however.
- In Williams v Nye (1890) 7 RPC 62 the patent was for a sausage machine that was a combination of a known mincing machine and a known skin-filling machine. Kekewich J held that the patent was invalid, and his decision was affirmed by the Court of Appeal on the ground that, although the claimed machine was new, it was not inventive.
- In British Celanese Ltd v Courtaulds Ltd (1935) 52 RPC 171 the main patent was for a process of manufacturing artificial silk. Clauson J held that the patent was invalid, and his decision was affirmed by both the Court of Appeal and the House of Lords. In the House of Lords it was common ground that the patented process consisted of four features, each of which was old. Lord Tomlin set out at 193 the legal proposition relied upon by counsel for the plaintiffs:
“It is accepted as sound law that a mere placing side by side of old integers so that each performs its own proper function independently of any of the others is not a patentable combination, but that where the old integers when together have some working inter-relation producing a new or improved result then there is patentable subject-matter in the idea of the working inter-relation brought about by the collocation of the integers.”
Lord Tomlin evidently agreed with this statement of the law, but he rejected the plaintiffs’ case on the facts at 194:
“In truth and in fact there is no inter-related working between the integers in the sense that any one of the integers is doing something which it could not do without the presence of one or more of the others. Each integer is fact performing its own part and is not functionally dependent upon the presence of any other integer at all. I think therefore that the invention lacks subject-matter.”
- In SABAF SpA v MFI Furniture Centres Ltd [2004] UKHL 45, [2005] RPC 10 the patent was directed to burners for separate gas hobs which took up as little vertical space as possible. In gas cookers and hobs the gas had to be mixed with air before it was ignited in order to burn steadily. In addition, the pressure of the gas had to be sufficient to expel it through the holes in the burner in a steady stream. In gas cookers both requirements were met by the use of a tube which passed horizontally below the hob and then turned upwards to connect with the burner. The tube had an air inlet. It also had a slight flare which, by virtue of the Venturi effect, increased the pressure of the mixed gas and air. The disadvantage of this arrangement was that it took up vertical space. The invention achieved a more compact hob by an arrangement in which both the air intake and the Venturi effect took place above, instead of below, the hob.
- At trial Laddie J held that:
“… the two important features of the SABAF burners which are said to constitute an invention are (i) drawing primary air in from above the hob unit and (ii) the use of a flow path under the flame spreader in which the Venturi effect will be present [a ‘radial’ Venturi]. … there is nothing in the specification to suggest, nor has it been seriously argued, that these two features interact with each other.”
He went on to find that two of the cited items of prior art made it obvious to have an air intake above the hob and another two items made it obvious to use a radial Venturi, but there was no item of prior art which taught both features. He nevertheless held that the patent was invalid on the ground that the claimed invention was a collocation of two features each of which was obvious, citing Lord Tomlin in British Celanese and the Guidelines for Examination in the European Patent Office.
- The relevant passages in the current (March 2021) edition of the Guidelines are little changed from those cited in SABAF. They state:
“Part G Chapter VII - Inventive step
…
5.2 Formulation of the objective technical problem
…
Sometimes, the objective technical problem must be regarded as an aggregation of ‘partial problems’. This is the case where there is no technical effect achieved by all the distinguishing features taken in combination, but rather a plurality of partial problems is independently solved by different sets of distinguishing features (see G-VII, 6 …).
…
6. Combining pieces of art
…
A different situation occurs where the invention is a solution to a plurality of independent ‘partial problems’ (see G-VII, 7 and 5.2). Indeed, in such a case it is necessary to separately assess, for each partial problem, whether the combination of features solving the partial problem is obviously derivable from the prior art. …
…
7. Combination vs. juxtaposition or aggregation
The invention claimed must normally be considered as a whole. When a claim consists of a ‘combination of features’, it is not correct to argue that the separate features of the combination taken by themselves are known or obvious and that ‘therefore’ the whole subject-matter claimed is obvious. However, where the claim is merely an ‘aggregation or juxtaposition of features’ and not a true combination, it is enough to show that the individual features are obvious to prove that the aggregation of features does not involve an inventive step (see G-VII, 5.2, last paragraph). A set of technical features is regarded as a combination of features if the functional interaction between the features achieves a combined technical effect which is different from, e.g. greater than, the sum of the technical effects of the individual features. In other words, the interactions of the individual features must produce a synergistic effect. If no such synergistic effect exists, there is no more than a mere aggregation of features …
Annex
…
2.1 Obvious and consequently non-inventive combination of features:
The invention consists merely in the juxtaposition or association of known devices or processes functioning in their normal way and not producing any non-obvious working inter-relationship.
Example: Machine for producing sausages consists of a known mincing machine and a known filling machine disposed side by side.”
- The Court of Appeal reversed Laddie J’s decision on the basis that it was impermissible to combine two prior art disclosures unless it was obvious to do so, and Laddie J had not held that it would be obvious to the skilled person to combine the teaching of either of the first pair of citations with either of the second pair of citations.
- The House of Lords reversed the Court of Appeal. Lord Hoffmann said at [24]:
“In my opinion the approach of the Court of Appeal is contrary to well established principles both in England and in the European Patent Office, as stated in the quotation from Lord Tomlin and the EPO Guidelines to which I have referred. I quite agree that there is no law of collocation in the sense of a qualification of, or gloss upon, or exception to, the test for obviousness stated in s.3 of the Act. But before you can apply s.3 and ask whether the invention involves an inventive step, you first have to decide what the invention is. In particular, you have to decide whether you are dealing with one invention or two or more inventions. Two inventions do not become one invention because they are included in the same hardware. A compact motor car may contain many inventions, each operating independently of each other but all designed to contribute to the overall goal of having a compact car. That does not make the car a single invention.”
- Lord Hoffmann went on:
“26. The EPO guidelines say that ‘the invention claimed must normally be considered as a whole’. But equally, one must not try to consider as a whole what are in fact two separate inventions. What the Guidelines do is to state the principle upon which you decide whether you are dealing with a single invention or not. If the two integers interact upon each other, if there is synergy between them, they constitute a single invention having a combined effect and one applies s.3 to the idea of combining them. If each integer ‘performs its own proper function independently of any of the others’, then each is for the purposes of s.3 a separate invention and it has to be applied to each one separately. That, in my opinion, is what Laddie J. meant by the law of collocation.
27. If one approaches the matter on this basis, it is clear that Laddie J. correctly applied the relevant principles at each stage. He found that taking the air above the hob and having a radial Venturi had no effect upon each other and that he was therefore dealing with two alleged inventions, each of which had to pass the test laid down in s.3 . He identified the inventive step in each. He asked himself what in each case were the differences between the relevant prior art and the invention. He found that there were virtually none. He concluded that it would have required no invention on the part of the skilled man armed with common general knowledge in the art to design a product in accordance with the alleged invention. In other words, he applied s.3 according to the Windsurfing structure to each of the features alleged to constitute the invention.”
- In Degussa-Huls SA v Comptroller-General of Patents [2004] EWHC 3213 (Pat) [2005] RPC 29 Pumfrey J expressed the view at [33] that SABAF was probably mainly concerned with claims to mechanical inventions. In the present case, however, the judge held at [469] that the collocation principle was a general one, and so it was capable, as a matter of law, of applying to an invention consisting of chemical molecules. Illumina does not contend that he was wrong about that.
- In Abbott v Evysio (cited above) Kitchin J held at [182]:
“… The first step is to determine whether the claim is concerned with a single invention or not. If two integers interact upon each other, if there is synergy between them, they constitute a single invention having a combined effect. If each integer performs its own proper function independently of the others then each is a separate invention and can be considered as such for obviousness purposes.”
- He went on to conclude at [185]:
“In the light of the evidence I am satisfied that these patents cannot be considered as simply a collocation of elements which perform their own functions independently of each other. There is an interaction between them which the designer of the stent must take into consideration. Each element cannot be regarded as an individual invention for obviousness purposes.”
- There was some debate on the appeal in the present case as to how the court should decide whether the claim consists of one invention or a plurality of inventions. In the end I understood it to be more or less common ground that this was primarily a question of what the patent disclosed, read in the light of the common general knowledge, but that evidence was admissible either to show that an interaction between features claimed in the specification did not occur or to show that an interaction not spelt out by the specification would be apparent to the skilled person or team.
- The judge’s reasoning. The judge began by noting two points which were not determinative of the issue:
“504. MGI point to the fact that the 415 patent is written on the basis that the claimed molecule is made up of building blocks - dye, linker, nucleotide etc. That is true but while it gives some support to the argument, in the end it is not determinative. Almost all inventions can be described as being made up of parts but that is not an admission by the patentee that the parts form a mere collocation.
- He went to hold that the claimed invention was a single invention rather than a collation of two features for the following reasons:
“510. As a matter of fact the claimed thing is a single molecule. The evidence is clear that these two aspects of that molecule are capable of interacting with one another. There is a potential for interaction between these aspects which the skilled person must always take into consideration. The fact the interaction would be one which is unhelpful does not mean it is not relevant. Moreover in this, essentially empirical, field the skilled person will not know whether or not there is in fact an interaction until a test is done. In fact the tests are not burdensome, but they would need to be done. In that sense this is a long way from Sabaf because there is no basis in that case for thinking there might be an interaction and then looking to find out. The two aspects in Sabaf simply do not interact with one another. The skilled person did not have to test them to find out. …
…
514. Putting it another way, the molecule of claim 1 is a single invention. Its beneficial properties derive from the functional relationship, which includes non-interference, between the constituent parts. I find that claim 1 of the 415 patent is valid.”
- The appeal. MGI’s case on the appeal is simple and straightforward. MGI point out that the judge made no finding that the two elements of claim 1, namely (i) the 4 carbon linker form of dye XVI and (ii) a derivatised nucleotide plus linker LN3, combined to produce a synergistic effect or otherwise interacted in any non-obvious way. No such interaction is suggested in the specification, whereas [0037] and Example 9 do suggest an interaction where a PEG spacer is used; nor did the judge find that the skilled team would think that there was any such interaction. Rather, the judge’s reasoning was that the skilled team would appreciate from their common general knowledge that the two elements had the potential to interact with each other in an unwelcome manner, but the specification showed that in fact they did not. MGI’s submission is that that is not enough to save the claimed invention from being a mere collocation of uninventive features.
- Attractively though this argument was presented by counsel for MGI, I do not accept it. I agree with the judge that, even assuming that the collocation principle is applicable to an invention consisting of a class of molecules, the application of the principle must take account of that technical context. Although the molecules claimed in the 415 Patent are made up of building blocks, those building blocks are incorporated into single molecules. The judge found that the skilled team would know from their common general knowledge that, when joined together, these building blocks were capable of interacting adversely with each other in various ways and that the skilled team could not predict in advance whether this would occur or not. If it did occur, the claimed molecules would not be useful in sequencing by synthesis. The 415 Patent demonstrates that there is no such adverse interaction, and thus the claimed molecules are useful for that purpose. It follows that the 415 Patent claims a single invention that makes a technical contribution to the art which neither Milton nor Arnost makes even when taken together. If it was obvious to the skilled team to combine the teaching of Milton with the teaching of Arnost, then the claimed invention would be obvious; but the judge found that that was not obvious, and there is no appeal against that finding.
Conclusion
- For the reasons given above I would also dismiss MGI’s appeal in respect of the 415 Patent.
Lord Justice Nugee:
- I agree.
Lord Justice Warby:
- I also agree.




 ”
”




 ”
”







 ”
”
