This Judgment was handed down remotely by email circulation to the parties’ representatives and release to the National Archives. Deemed date for hand-down: 1st June 2022.
Mr Justice Meade:
Introduction. 3
The issues. 3
The witnesses. 4
The law - obviousness. 6
Basic principles. 6
Brugger v. Medicaid. 6
Inhale v Quadrant 7
Conor v. Angiotech. 7
Relying on problems not solved. 8
The skilled team - the law.. 9
The skilled team - assessment 10
Agreed common general knowledge. 11
Bladder Physiology. 12
Storage phase. 13
Voiding phase. 14
Treatment Of Overactive Bladder Syndrome/Its Constituent Symptoms. 15
Methods Of Investigating New Therapies. 15
In vitro methods: 15
In vivo methods: 16
β3 adrenoceptors. 17
Disputed common general knowledge. 18
The β3 adrenoreceptor in detrusor function and β3-AR agonists. 18
CIR/SWOT. 24
Other therapeutic approaches. 25
The teaching of the Patent 28
The claims of the Patent 29
Obviousness over `288. 30
Teaching of `288. 30
Which compounds are disclosed as having been tested and for what?. 34
Obviousness discussion. 35
Pozzoli steps 1 and 2. 35
Pozzoli step 3. 35
Pozzoli step 4. 35
The parties’ key points. 35
Key cross-examination relied on. 37
Urine retention. 40
Assessment 40
The insufficiency squeeze. 42
Conclusions. 43
1. In these two actions Teva Pharmaceutical Industries Limited (“Teva”) and Sandoz AG (“Sandoz”) seek the revocation of European Patent (UK) 1 559 427 B1 (“the Patent”) in the name of Astellas Pharma Inc. (“Astellas”). The priority date, unchallenged by the start of trial, is 7 November 2002.
2. The Patent protects the compound mirabegron, which is sold by Astellas under the name Betmiga. It is a treatment for overactive bladder (“OAB”). It is a β3 adrenoreceptor agonist (for short, I will in general refer to “β3-AR agonist” or “β3-AR agonism” in this judgment, although not when citing or quoting other documents).
3. There is also a corresponding SPC (SPC/GB13/035). The only attack on its validity is that the Patent is invalid so I need say no more about it separately.
4. Astellas has counterclaimed for infringement. Infringement is admitted by Teva and Sandoz (and in each case another group company the subject of a Part 20 claim, on whose identity nothing turns) in the event that the Patent is valid. So in substance this was a patent revocation trial.
5. Teva and Sandoz have distinct teams of solicitors at the same firm (owing to Sandoz’s team moving during the litigation). They use the same leading Counsel, but different junior Counsel. Mr Turner QC undertook all the oral advocacy for Teva and Sandoz, to whom I will refer collectively as “the Claimants”.
6. By the start of the trial, the issues were very limited:
i) A number of disputes over the skilled team.
ii) Some issues over common general knowledge (“CGK”).
iii) Obviousness over a single piece of prior art, Australian Patent Application AU 199889288 B2 (“`288”) published on 6 May 1999.
iv) Insufficiency, run as a squeeze against obviousness.
7. However, the limited scope of the issues was arrived at only very shortly before trial, and I need to say a little about the issues that were dropped, since it is necessary in order to understand some of the evidence and the roles of the respective experts.
8. Up until the day before the trial, and including in the Claimants’ opening, there was an attack on priority entitlement. In the event that priority was lost, certain other prior art citations were raised, some for novelty only.
9. After skeletons were submitted, the Claimants dropped their priority attack, and all of the prior art except `288.
10. Teva had also run a citation referred to as `111, which was a European application related to `288; `111 contains more examples than `288. A practical consequence of this was that much of Astellas’ evidence was written by reference to `111 rather than `288. The Claimants avoided that problem by their experts not dealing with `111, but regrettably they did not tell Astellas before experts’ reports. So when reading Astellas’ experts’ reports one has to have in mind that references to `111 can and generally should be read also as relating to `288.
11. I also record at this stage that `288 and `111 arise from a common PCT application, WO 99/20607 (“`607”), which is referred to in the Patent at [0004]. The evidence refers to `607 in places, but `607 is in Japanese and the parties treated `111 as being materially the same as `607.
12. The parties each called two experts, a clinician and a pharmacologist. There was no fact evidence.
13. The Claimants’ experts were:
i) Prof Paul Abrams (clinician);
ii) Dr Thomas Argentieri (pharmacologist).
14. Astellas’ experts were:
i) Dr Ian Mills (clinician);
ii) Dr Gordon McMurray (pharmacologist).
15. Dr Argentieri gave evidence by video platform from the US, which required splitting his evidence across two days to allow afternoon sittings, because of the time difference. I do not think this adversely impacted him or my ability to assess his evidence; a brief sound problem was cured during a break. All the other evidence and all the oral argument was live in Court. I am grateful, as ever, to the Court staff, the IT providers and the shorthand writers.
16. There was a noticeable difference between the sides’ respective expert teams in relation to the clinicians. Prof Abrams has had a distinguished career as a urology consultant in the NHS. Dr Mills on the other hand qualified as a medical doctor but since then has mainly worked in industry as a pharmaceutical clinician (including in particular at Pfizer). His professional accreditations make him the equivalent of a consultant in the NHS, but he has had much less patient-contact experience than Prof Abrams.
17. Both Prof Abrams and Dr Mills were well able to explain clinical matters of relevance to the case, but there was little dispute over the “pure” clinical picture. That and the fact that Prof Abrams’ written evidence was largely directed to issues arising on the priority attack meant that he spent little time in the witness box. Dr Mills was questioned for considerably longer, and that was because his perspective as a pharmaceutical clinician meant that his views overlapped with those of the pharmacologist more than those of Prof Abrams did. None of this is a criticism of either of them, but it feeds into a point about the skilled team, to which I return below.
18. In closing written submissions the Claimants said that while they made “no particular criticisms of any of the expert witnesses”, Dr Mills and “perhaps to a lesser extent” Dr McMurray held on to unsustainable positions “but not sufficiently to colour the overall value of their evidence”. They gave certain examples thereafter and I will touch on these where relevant, but there was nothing in them to lead me to doubt my overall impression that both gave their evidence, for which I am grateful, fairly and honestly. They were also both good explainers.
19. The Claimants also said in closing written submissions that Astellas’ experts “gave the appearance (we can say only appearance) of having been overly lawyered”. I do not think they gave any such appearance and anyway since the Claimants stopped short of saying that the witnesses in fact were “overly lawyered” I cannot see where the point would go.
20. No criticism was made of Prof Abrams, who was a good, clear, knowledgeable witness and entirely fair. His role in the case was fairly limited, for the reasons explained above.
21. Counsel for Astellas criticised Dr Argentieri on several fronts:
i) That he approached `288 on the basis that the skilled pharmacologist had already decided to pursue β3-AR agonists and that the relevant question was, leaving aside all other patents and molecules, “would it be unreasonable to synthesise all six compounds [in `288] and test them”;
ii) That he conceived of the skilled addressee as being “smarter than everyone else”;
iii) That he produced an incomplete list of the therapeutic pathways that were under consideration for OAB at the priority date;
iv) That he said `288 contained “data” when it in fact did not in the relevant respects.
22. The first two of these are not criticisms of Dr Argentieri’s independence or integrity, but rather go to whether he was asking himself the right question. I think there was some force in them and I deal with them where they arise but they do not lead me to conclude that he was not doing his best to give complete and honest evidence.
23. In relation to the third point I note that Dr Argentieri provided the list from memory and directed only to the pathways that he thought had momentum or a lot of recent attention at the priority date. I do think the list was materially incomplete nonetheless and Dr Argentieri should have taken more care, including by checking the literature. Questions put to Dr Argentieri about how papers for inclusion in his report were selected from a much larger number initially identified also gave me the impression that he had left the choice to the Claimants’ lawyers too much, rather than making his own independent decision.
24. The fourth point was portrayed by the Claimants as a semantic point on which they also said that Dr Argentieri was strictly correct. I do not agree; what Dr Argentieri said tended to overstate how convincing the content of `288 was. I was also a little concerned by his assertions (i) that the bare claim in `288 to selectivity was “compelling” and (ii) that the failure of β3-AR agonists for weight-related conditions was a positive because it tended to show that side effects were unlikely if used for OAB, neither of which Counsel for the Claimants could defend.
25. Overall these points left me with the impression that Dr Argentieri was trying a little too hard to find points in favour of the Claimants. It was not enough to lead me to reject his evidence outright, and many of his points were well made and solidly supported, but I bear it in mind and I thought that Astellas’ witnesses were overall more fair and balanced when it came to the issues on CGK and obviousness, and put themselves in the position of the ordinary uninventive addressees better than him.
26. There was no disagreement about the basics, but some argument on more detailed points.
27. It was common ground that the modern approach is as set out in Actavis v. ICOS [2019] UKSC at [52] - [73]. At [63] the Supreme Court endorsed the statement of Kitchin J as he then was in Generics v. Lundbeck [2007] EWHC 1040 (Pat) at [72], to which Counsel for the Claimants took me in the course of argument:
The question of obviousness must be considered on the facts of each case. The court must consider the weight to be attached to any particular factor in the light of all the relevant circumstances. These may include such matters as the motive to find a solution to the problem the patent addresses, the number and extent of the possible avenues of research, the effort involved in pursuing them and the expectation of success.
28. Brugger v. Medicaid [1996] RPC 635 (at 661) was approved by the Supreme Court in Actavis v. ICOS in its statement of principle that an obvious route is not made less obvious by the existence of other obvious routes. That does not mean, however, that the existence of a number of ways forward is irrelevant; the “number and extent of the possible avenues of research” was expressly called out as a relevant factor in Generics, as quoted above. The point made by Laddie J in Brugger was that once a route is obvious then it is not saved by other obvious possibilities. So a close scrutiny of the facts is necessary.
29. Counsel for the Claimants made a more subtle point, which was that usually the “possible avenues of research” relevant will be those leading from the cited prior art; a patentee will not usually be able to make much if anything of an argument that the existence of a totally different approach from another starting point different from the cited art means that it is not obvious to take the cited art forward. I think there is something to this, but it must depend on the facts and it cannot be a black and white rule. Counsel for the Claimants accepted that if the CGK was that treatment for OAB was a field where as a whole there was no clear way forward among multiple options, that could be a factor.
30. Counsel for Astellas stressed the principle from Inhale v Quadrant [2002] RPC 21 that although the skilled person is deemed to read each piece of prior art with interest, they do not come to it with the expectation that it will contain the answer to a problem they are faced with. I accept the principle, which I do not think Counsel for the Claimants really disputed, but its relevance to the present case is low, because if the main planks of the Claimants’ case were made good (that selective β3-AR agonists were understood to have excellent prospects to treat OAB and that `288 disclosed mirabegron to be a good and selective β3-AR agonist) then there would be no need for a synthetic or a priori expectation that `288 would be interesting.
31. In Conor v Angiotech [2008] UKHL 49 Lord Hoffmann rejected an argument that the invention should be treated as being merely the idea of trying a taxol-coated stent. He said the following:
16. On the basis that the patent taught no more than that taxol was worth trying, [Mr Thorley QC, Counsel for the party attacking the Patent] submitted that it added nothing to existing knowledge. It was common ground that taxol was, like many other anti-proliferative drugs, worth a try. And that was obvious. It was not necessary for Conor to show that it was obvious actually to use taxol to treat restenosis because the patent did not teach that it would work.
17. I shall say at once that in my opinion this argument was an illegitimate amalgam of the requirements of inventiveness (article 56 of the EPC) and either sufficiency (article 83) or support (article 84) or both. It is the claimed invention which has to involve an inventive step. The invention means prima facie that specified in the claim: see section 125(1) of the 1977 Act. In the present case, the invention specified in claim 12 was a stent coated with taxol. There was no dispute that this was a new product. The question should therefore simply have been whether it involved an inventive step. As in the case of many product claims, there was nothing inventive in discovering how to make the product. The alleged inventiveness lay in the claim that the product would have a particular property, namely, to prevent or treat restenosis. (Compare Pharmacia Corp v Merck & Co Inc [2002] RPC 775). So the question of obviousness was whether it was obvious to use a taxol-coated stent for this purpose. And this, as I have said, was the question to which the experts addressed themselves.
18. Mr Thorley, however, sought to avoid this question by watering down the claimed invention by reference to what he said were inadequacies in the specification. It did not contain information about human or animal tests which showed that it would work or provide enough information about doses and so forth to enable the skilled person to work it. It was therefore nothing more than an idea that taxol might work and any skilled person would have known that.
19. In my opinion, however, the invention is the product specified in a claim and the patentee is entitled to have the question of obviousness determined by reference to his claim and not to some vague paraphrase based upon the extent of his disclosure in the description.
…
32. The reason that I refer to this is that during the cross-examination it was apparent that the Claimants’ obviousness case was, in part, being advanced by reference to what were said to be limitations in the experimental proof in the Patent, and to a still greater extent, the Priority Document. This was confirmed by the Claimants’ closing written argument, which included several pages on what the Priority Document did and did not contribute, and then a comparison of the Priority Document with the Patent.
33. To the extent that such cross-examination elicited statements of technical opinion themselves relevant to the real issue of obviousness that is fair enough, but a lot of questions were along Conor lines - that the experiments in the documents were limited in what they proved compared to experiments in the prior art or CGK. I expect that this was largely a consequence of the priority issue dropping out so late, and after some discussion Counsel for the Claimants accepted that obviousness had to be assessed by reference to the claim, although he maintained that technical contribution and/or the problem solved remained relevant.
34. A patentee cannot rely for obviousness on problems deterring a course of action leading to that which is claimed, if the patent in question does not address the problems. This has been held in a number of cases; I was referred to Philips v. Asustek [2019] EWCA Civ 2230 (Floyd LJ with Patten and Henderson LJJ in agreement) at [73]:
73. Finally, the defendants contend that the issues which the judge held would have deterred the skilled person from proceeding to implement Shad at the base station remained issues for the implementation of the 525 patent, in the sense that the patent did not teach the skilled person how to overcome them. This is the point based on the passage from Pozzoli which I have cited above. The principle is that you cannot have a patent for doing something which the skilled person would regard as old or obvious but difficult or impossible to do, if it remains equally difficult or impossible to do when you have read the patent. To put it another way, the perceived problem must be solved by the patent.
35. In the present case there is no doubt that the skilled team in this field would have a keen awareness of the likelihood and risks of side effects with any mechanism, including β3-AR agonism. A main potential cause of side effects for a β3-AR agonist under consideration would be off-target effects if the compound turned out to be an agonist of β1 or β2 as well and the skilled team might be deterred from proceeding with a compound whose selectivity was unknown. But since the Patent contains nothing to say whether or to what extent mirabegron was selective for β3 over β1 and β2, Astellas cannot rely on this, as its Counsel accepted. Astellas did argue that urine retention would be a side effect of concern, and it did so on the basis that the Patent does address it. That point therefore depends on the facts.
36. There was no dispute about the basic notion of the skilled addressee as being a person with a practical interest in the subject matter of the patent under consideration, possessed of the common general knowledge, and diligent but uninventive. Nor was it in dispute that the addressee may be a team, and would be in the present case.
37. There was however a dispute on the facts of the present case about the breadth of interest, or degree of specialisation, of the skilled team in the present case. I have dealt with this in a number of recent cases, by reference to my decision in Alcon v. Actavis [2021] EWHC 1026 (Pat) drawing on the decision of Birss J, as he then was, in Illumina v. Latvia [2021] EWHC 57 (Pat).
38. In Illumina Birss J provided the following approach, from [68] on:
“68. I conclude that in a case in which it is necessary to define the skilled person for the purposes of obviousness in a different way from the skilled person to whom the patent is addressed, the approach to take, bringing Schlumberger and Medimmune together, is:
i) To start by asking what problem does the invention aim to solve?
ii) That leads one in turn to consider what the established field which existed was, in which the problem in fact can be located.
iii) It is the notional person or team in that established field which is the relevant team making up the person skilled in the art.”
39. And in Alcon at [31] I said:
“31. I intend to apply that approach. I take particular note of:
i) The requirements not to be unfair to the patentee by allowing an artificially narrow definition, or unfair to the public (and the defendant) by going so broad as to “dilute” the CGK. Thus, as Counsel for Alcon accepted, there is an element of value judgment in the assessment.
ii) The fact that I must consider the real situation at the priority date, and in particular what teams existed.
iii) The need to look for an ‘established field’, which might be a research field or a field of manufacture.
iv) The starting point is the identification of the problem that the invention aims to solve.”
40. There are three aspects to the assessment of the skilled team that I need to mention, although as I shall explain the first two largely fizzled out, or seemed to me, on analysis, not to matter much if at all.
41. The first is a relatively simple one about which in the end I do not think there was a material dispute. It relates to the role of the clinician and it really only arises because, as I have explained above, the Claimants called Prof Abrams, a “pure” clinician, while Astellas called Dr Mills, a pharmaceutical clinician from industry. I thought this was inconsequential because it was common ground that the clinician would need to understand the therapeutic area and the relevant basic science, and both experts were able to cover this. Someone like Dr Mills would interface more closely and more continuously with the pharmacologist, and they would probably work for the same company in real life. In practical terms at trial, the result was that Dr Mills’ and Dr McMurray’s evidence overlapped rather, although not problematically. This difference might also mean that the “pure” clinician would be involved at the start of a project to identify and shape the clinical requirements and, if the project were successful, later on when clinical trials needed to be planned and executed, but not in between. Astellas said that this was odd, but I do not see why. In any event, it does not make a difference. I agree with what Henry Carr J said in Fujifilm v. AbbVie [2017] EWHC 395 (Pat) at [118]-[119] that this kind of debate is pointless because the skilled team is a notional construct and what matters is the overall skill-set of the team, not the precise distribution of it among the team’s members.
42. The second was which of the two experts would be the “leader” of the team. Although Henry Carr J in Fujifilm said that there was no dispute in that case that the rheumatologist would lead the team, in Halliburton v Smith [2006] EWCA Civ 1715 at [14] Jacob LJ said that the notional team “… is a team with no boss. Each member of the team is assumed to play his/her own part”. I do not think these are inconsistent, partly because Henry Carr J went on to say that it did not matter, but also because it is often possible to say from which member of the skilled team the initiation of a project might come without passing judgment as to which would give orders to the other. In the present case, the situation was really a clinical problem of an effective class of drugs (anti-muscarinics) with unacceptable side-effects for the patients. This was not in dispute before me, so this point also does not matter.
43. The third aspect was the only one where there was a real dispute and it relates to the Illumina kind of issue. The Claimants submitted that the notional skilled team would be a team working on β3-AR agonists for OAB. Were that the case, it would have assisted the Claimants because it would tend to help to side-line other pathways being researched for OAB.
44. To address this dispute, I have to decide, in the light of the legal principles mentioned above, what the relevant “established field” was in which the problem addressed by the Patent lay, with regard to real teams.
45. The Claimants pointed to a considerable number of teams interested in OAB and who were working on β3-AR agonism; they included academic teams as well as pharmaceutical companies. The Claimants also relied on the Yamaguchi paper accepted to be CGK (see below).
46. However, there was also a consistent pattern of evidence that teams in this field would choose a number of targets to work on to try to improve OAB treatments, and while the choice of targets of some teams would include β3-AR agonism, others would not. Nor, Counsel for the Claimants accepted, was there evidence of teams who only worked on β3-AR agonism. Obviously there were some publications that only covered β3-AR agonism, but they would come from teams with broader interests who, as part of that work, had some β3-AR agonist results to report (such as was the case with Dr Argentieri’s own group). I also take into account the early stage of β3-AR agonist work: there were no licensed β3-AR agonist drugs and understanding of the possibilities and problems was still developing.
47. In addition, there were a number of review articles which looked at possible future treatments for OAB in the round, covering numerous mechanisms.
48. All these points lead me to conclude that the established field was broader than β3-AR agonism for OAB. It was, rather, new or improved pharmacological treatments for OAB generally. This means that the other possible approaches for OAB can potentially enter the picture as part of the CGK, and as a potential factor in relation to obviousness.
49. The parties helpfully provided a statement of agreed CGK. I reproduce it here with minor editing, in particular the deletion of a section about the terminology applied to OAB and related conditions, which was relevant to priority and no longer mattered at trial. It was not wrong, or disputed, just no longer of importance. I have also slightly changed the introduction of the Yamaguchi paper in this section because of the acceptance by Astellas that it was itself CGK.
50. The lower urinary tract in humans consists of the urinary bladder and the urethra. The bladder is a hollow, muscular organ which stores urine and is divided into its two main parts: the body and the base. Urine enters the bladder from the kidneys via the ureters. The bladder body is mainly comprised of a muscular wall with smooth muscle cells, referred to as the detrusor muscle, which is by far the largest part of the bladder. The bladder base consists of the trigone and the bladder neck, which leads to the urethra in the wall of which the urethral sphincter is embedded.
51. The smooth muscle in the detrusor is structurally and functionally different from the muscles found in the bladder base, the urethra and the pelvic floor. Within the wall of the urethra, just above the pelvic floor, is the intraurethral (also termed intramural) striated muscle sphincter which prevents urine leakage during filling and relaxes to allow the bladder to empty.
52. The urethra is the conduit through which urine flows during voiding. It passes through the pelvic floor muscles and comprises both striated and smooth muscles. Together, the striated muscle and smooth muscle form the urethral sphincter mechanism, whose contraction during urine storage causes increased resistance in the urethra which prevents urine leakage. In men, the urethra is about three times the length of the female urethra, largely due to the extra-pelvic extension of the urethra along the length of the penis. In the male the proximal urethra, which is within the pelvis, is surrounded by the prostate gland.
53. The following Figure shows a schematic representation of the human lower urinary tract.
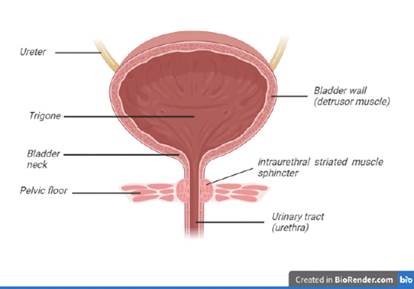
54. The main functions of the bladder are to store urine as it flows from the kidneys into the bladder during the ‘storage phase’ and to rapidly empty the urine during the act of urination, also known as micturition or voiding, which is referred to as the ‘voiding phase’. In a healthy individual, mechanoceptors respond to stretching of the bladder wall and give rise to the sense of wanting to urinate, alerting the person of the need to empty their bladder.
55. Under normal physiological conditions, soon after urination, the bladder neck closes, the muscles of the pelvic floor and the urethral sphincter contract (tighten), and the bladder body relaxes to allow the bladder to fill with urine from the kidneys. The bladder expands at low pressure through a special mechanism (which allows it to expand without increasing internal pressure in the bladder), so that there is no back pressure effect on the kidneys.
56. The interactions of the anatomical features of the lower urinary tract and the human nervous system comprise a tightly-controlled feedback loop mechanism involving the brain, the spinal cord, peripheral nerves and the lower urinary tract. The lower urinary tract was known to be innervated by peripheral nerves of the parasympathetic and sympathetic branches of the autonomic nervous system (ANS), and by the somatic nervous system.
57. The ANS is a division of the peripheral nervous system; it acts mostly unconsciously and regulates bodily functions such as breathing, digestion and urination. The parasympathetic and sympathetic branches of the ANS essentially act in opposition to one another. Put simply, the sympathetic nervous system is active during the storage phase and the parasympathetic nervous system is active during the voiding phase.
58. The somatic nervous system is associated with the voluntary control of movement through skeletal muscle (it is also known as the voluntary nervous system), as well as involuntary control via reflexes. The somatic nerves innervate the striated muscles of the pelvic floor and the urethral sphincter and are active during bladder filling to maintain continence.
59. The autonomic and somatic nervous systems exert their control through chemical messengers known as neurotransmitters. The relevant neurotransmitters are acetylcholine (“ACh”), and noradrenaline (also known as norepinephrine).
60. The Skilled Team would have been aware of the following mechanisms that regulate the storage and voiding phases:
61. Sensory nerve signals are continually and increasingly generated from the bladder and pass up the spinal cord to the brain (via autonomic and somatic nerves, referred to as ‘afferent innervation’). Under normal physiological circumstances, the micturition reflex is continuously and unconsciously inhibited during filling.
62. There are no signals from the parasympathetic nervous system to the detrusor, and therefore no contraction occurs. Activation of the sympathetic nerves triggers the release of noradrenaline which binds to adrenoceptors causing the detrusor to relax. Noradrenaline is also released in the smooth muscle of the urethral sphincter where it binds to α1 adrenoceptors, causing contraction.
63. The somatic nerves innervating the striated muscles of the pelvic floor and the urethra release ACh triggering them to remain tightened and closed.
64. In this manner pressure in the bladder remains low whilst pressure in the urethra remains high, allowing urine storage.
65. The bladder is under a person’s voluntary control when it comes to voiding. When s/he decides to void, nerve impulses travel from the brain and down the spinal cord to the lower urinary tract through the peripheral nerves.
66. The somatic nerves are inhibited, as is the sympathetic outflow to the bladder base and the urethral smooth muscle, to allow relaxation of the bladder outlet and pelvic floor. The parasympathetic nerves that supply the detrusor release ACh, which stimulates muscarinic receptors (a sub-class of the cholinergic receptors) leading to detrusor contraction.
67. In the voiding phase pressure in the bladder increases whilst the pressure in the urethra is reduced, allowing urine to flow out of the bladder.
68. The following figure shows the above (with the exception of the sympathetic nervous system innervation of the smooth muscle of the urethral sphincter) in a basic schematic form (not to scale).
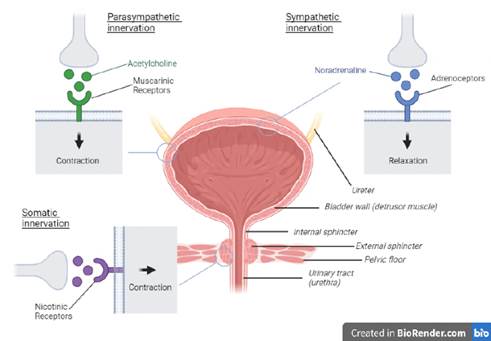
69. The initial management of overactive bladder syndrome was with lifestyle changes (for example, altering fluid intake or caffeine intake of patients) and behavioural therapies (such as through pelvic floor muscle training and bladder re-training, where the patient records their urinary diary and is asked to progressively extend the period without urination). If these initial steps were not adequately successful, antimuscarinic drugs were prescribed. Existing antimuscarinic drugs at the Priority Date included oxybutynin hydrochloride, tolterodine tartrate, flavoxate hydrochloride, and propiverine hydrochloride. If the patient remained dissatisfied with their progress, they would be referred for specialist care including UDS, prior to interventional treatment such as neurostimulation or sacral blockade.
70. Antimuscarinics were the frontline existing pharmaceutical treatment at the Priority Date; they work by blocking muscarinic receptors, preventing binding of ACh and therefore impeding detrusor contraction. It was well known that the existing antimuscarinic compounds had significant unwanted side-effects caused by “off-target” responses at receptors elsewhere in the body (i.e., not on the detrusor muscle).
71. Known side-effects of antimuscarinics included dry mouth, blurred vision and difficulty with visual accommodation, constipation, and tachycardia, leading to low patient compliance. Studies had shown that dry mouth was considered to be particularly troublesome and was the most common side effect complained of by patients.
72. Further, antimuscarinics inhibit contraction of the bladder and it was known that this can include inhibiting normal contraction of the bladder (i.e., in the voiding phase when the patient wishes to urinate). Inhibition of normal contraction leads to incomplete voiding and an increased residual post-void volume; in other words, the bladder never properly empties, and urine is retained in the bladder after urination.
73. Despite the well-known problems referred to above, antimuscarinics were still the front-line treatment at the Priority Date. As a result there was a strong interest in the development of new drug treatment options. Various targets were under consideration, exactly how many, which and their associated CGK is disputed and therefore appears in the list of disputed CGK issues; given the relevance of β3 adrenoreceptors to the present dispute these are discussed in more detail below.
74. Methods of pre-clinical research into lower urinary tract function included in vitro and in vivo experimental approaches.
75. In the organ bath methodology, strips of detrusor muscle (derived from a variety of different animal species, and from humans) are dissected, suspended under tension in an organ bath and perfused with physiological saline. This method could be used to evaluate the pharmacological effects of potential therapies on contraction of detrusor muscle strips. Carrying out this process in the absence and then in the presence of a potential agent may be useful in demonstrating that the agent prevents the contraction of the bladder or causes relaxation. In comparison to live models, a bladder strip assay has the limitation of being outside of the influence of the rest of the body (e.g., the effects of the nervous system).
76. Potential new therapies can be investigated using artificial filling cystometry in healthy and disease model animals (i.e. physiological or pathological models). Artificial filling cystometry involves cannulating the animal’s bladder, allowing it to be artificially filled and the bladder’s response measured. This can be used to assess bladder function in a variety of different species by measuring the pressure and volume of liquid in the bladder during the storage (filling) and voiding phases as well as frequency of bladder contraction and/or micturition, voided volume and residual post-void volume (residual urine left in the bladder after micturition may indicate that normal contraction has been inhibited).
77. Physiological models allow one to investigate the effects of a compound on bladder physiology, but do not recreate or mimic the pathology of interest. As these models do not mimic a pathological condition they can only demonstrate the activity of a compound on normal physiology, not the efficacy of the compound on abnormal physiology. Pathological models seek to mimic the relevant pathological condition and can produce data which may be more relevant to the disease state.
78. One CGK example of a pathological model is a bladder hypertrophy model, which seeks to mimic a pathological situation where bladder outlet obstruction causes the bladder to hypertrophy. This requires the animal’s urethra to be narrowed with a ligature so that it is tight enough to create a blockage, but not so tight so as to cut off urine flow through the urethra entirely. The obstruction leads to bladder hypertrophy as the bladder acts to overcome the obstruction. This leads to an unstable, hypercontractile bladder, manifesting as increased bladder contractile activity during filling. By partially obstructing bladder outflow, hypertrophy and hypercontractility is induced.
79. A second CGK example of a pathological model uses acetic acid or a similar compound (an example of an alternative compound is cyclophosphamide). Acetic acid hyper-activates sensory neurons in the bladder, leading to abnormal contractility. Hyperactive sensory neurons are implicated in OAB. By administering acetic acid into the bladder, the sensory neurons become hyper-activated resulting in abnormal contractility which somewhat mimics detrusor overactivity.
80. It is not possible to directly assess whether a compound has an effect on urgency using pre-clinical in vitro or in vivo measures.
81. The experimental techniques described in Examples 1, 2 and 3 of the Patent were part of the common general knowledge.
82. β3 adrenoceptors are G-protein coupled receptors, so called because the major intracellular signalling protein family with which they interact are the 'G-proteins' (although other interactions are known to occur and can result in the activation of additional intracellular pathways).
83. As of the Priority Date it was known that the β adrenoceptor family included β1, and β2 adrenoceptors and it had recently been determined that ‘atypical’ adrenoceptors reported in earlier research were in fact a third sub-class, β3 adrenoceptors. β1 adrenoceptors were known to be located predominantly on cardiac muscle, mediating increased heart rate and force of contraction. β2 adrenoceptors were known to be located predominantly on smooth muscles mediating relaxation, especially in blood vessels where they mediated vasodilatation, in the lung where they mediated bronchodilatation, and in the uterus where they mediated uterine relaxation. β2 adrenoceptor activation was also known to elicit tremors in humans due to activity at the level of skeletal muscle.
84. It was thought that the main β adrenoceptor found in the human detrusor was the β3 adrenoceptor. β3 adrenoceptors were also known to be present in fat cells. β3 adrenoceptor agonists were the subject of some human and animal in vitro and animal model research in relation to their effect on detrusor function.
85. The review article by Yamaguchi entitled 'β3-adrenoceptors in Human Detrusor Muscle' ("Yamaguchi") was CGK.
86. The following matters regarding β3 adrenoceptors were known:
i) that β3 adrenoceptors had recently been identified to be present in the human detrusor muscle via mRNA expression studies and to be the predominant β adrenoceptor in that tissue (but that β1 and β2 mRNA was also expressed);
ii) that a number of β3 adrenoceptor agonists which were thought to be selective were known, including BRL 37344, CL 316243, FK 175, CGP-12,177A and L-755,507 (which the Yamaguchi paper states to have a >1000-fold greater selectivity for the β3 receptor as compared to the β1 receptor and no activity at the β2 receptor);
iii) that β3 adrenoceptors were thought to be able to mediate relaxation of the detrusor based on experiments using isolated detrusor strips and selective β3 agonists and antagonists. The tissues were either taken from lab animals or, where human tissue was used, from patients who had undergone a cystectomy (removal of the bladder), likely necessitated by bladder cancer;
iv) that in vivo studies in animals (including rats with a urinary frequency phenotype) had demonstrated the ability of some β3 adrenoceptor agonists to increase bladder volume in a dose dependent manner, but also that relaxation of the detrusor of many species was thought to be mediated by β2 adrenoceptors in addition to the putative role of the β3 adrenoceptor;
v) that β3 adrenoceptors were known to be present in fat cells and the GI tract (where they were thought to regulate motility) and β3 adrenoceptor agonists had previously been tried as anti-obesity treatments in human clinical trials, based on demonstration of anti-obesity effects in animal models, that these studies were ultimately unsuccessful and revealed several issues impeding translation of the effects seen in nonclinical experiments to clinical efficacy, including that:
vi) the agonists tested had side effects of tremor and tachycardia (likely due to effects on the β1 and β2 adrenoceptors);
vii) the rat and human β3 adrenoceptors differ materially in their pharmacology such that agonists which were selective for the rat β3 adrenoceptor were not full agonists of the human receptor; and
viii) the results of clinical trials of β3 agonists for this indication (anti-obesity) had been weak or negative.
87. Although not explicitly called out in the agreed CGK document, it was also common ground that because OAB is not a condition which is life-threatening, but rather which causes inconvenience and discomfort (sometimes to a severe degree), the tolerance for side-effects with potential treatments was low.
88. The parties also provided a list of disputed CGK topics. There were originally seven but one fell away with the priority issue, and three proved to be of no relevance. The remaining three topics concerned (i) the CGK knowledge of the β3 adrenoreceptor in detrusor function, (ii) the techniques known as “CIR” or “SWOT” and (iii) the therapeutic approaches to OAB being researched at the priority date. Topics (ii) and (iii) overlap and interact, for reasons I will address below.
89. The disagreement on this topic was more one of emphasis than a black/white one.
90. It is useful, and was part of the Claimants’ approach, to give some historical perspective to this. Key points are as follows (I have only given illustrative citations, a number more were covered in the written and oral evidence):
i) In 1998, the possibility was identified that the β3-AR might be the subtype that mediated relaxation of human detrusor muscle (Igawa et al, “Possible β3-adrenoreceptor-mediated relaxation of the human detrusor”);
ii) In 1999, the presence of β3-AR in human detrusor tissue was identified (Igawa et al, “Functional and molecular biological evidence for a possible β3-adrenoreceptor in the human detrusor muscle”);
iii) Also in 1999, the expression of mRNA for rat and human β3-ARs in detrusor tissue was found to be increased, and in rat models detrusor muscle was relaxed by the use of a β3-AR agonist, and bladder capacity was increased with no change of micturition pressure or threshold pressure (Fujimura et al, “Expression and possible functional role of the β3-adrenereceptor in human and rat detrusor muscle”);
iv) Papers like Fujimura began to propose the use of β3-AR agonists in treating OAB, as did review articles from leading figures in OAB research such as Prof Karl-Erik Andersson at Lund University (“Treatment of overactive bladder: other drug mechanisms”, 2000);
v) In about 2001 it was reported that β3-AR agonists could have relaxant effect in human bladder detrusor tissue (Igawa, “Relaxant effects of isoprotenerol and selective β3-adrenereceptor agonists on normal, low compliant and hyperreflexic human bladders”).
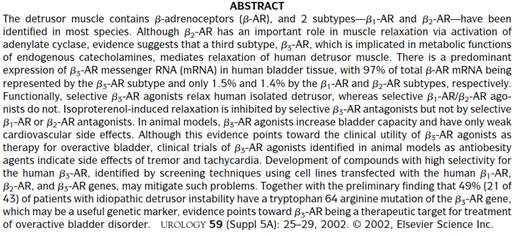
91. The overall position reached, and aspects of the above developments, were summarised in Yamaguchi, which, as I have already said, was agreed to reflect, and itself to be, CGK.
92. In view of the importance attached to it, I will quote some key passages from Yamaguchi. Its abstract was as follows:
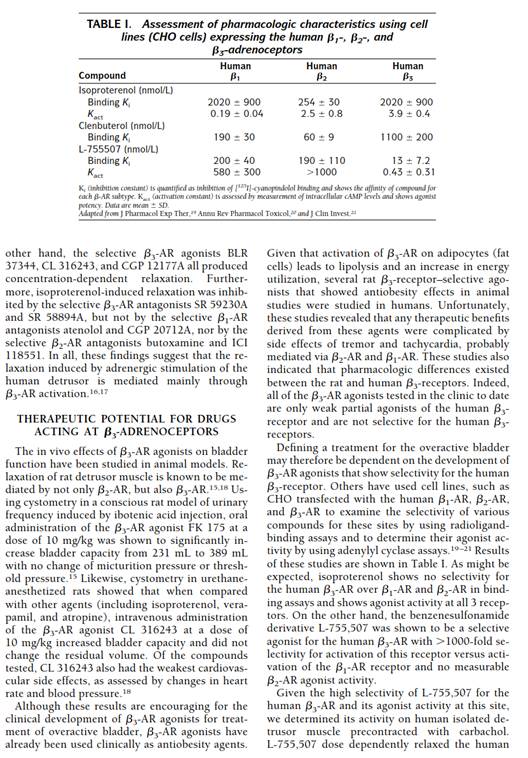
93. At pages 26 to 28, it said as follows (picking it up from the last paragraph on page 26, rhc):

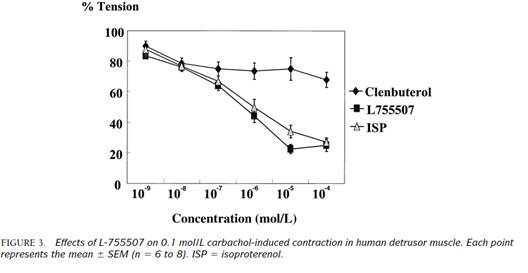

94. The conclusion was as follows:
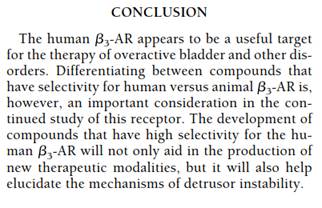
95. Yamaguchi has a section called “Consequences of β3-adrenoreceptor gene mutation” on page 28. Although agreeing that the paper generally was CGK and of high quality, Dr McMurray said that the sample size in this section was too small to support its conclusions. His point sounded reasonable but it was not explored with Dr Argentieri so I do not feel in a position to decide it. In oral closing submissions Counsel for the Claimants did not identify, when asked, any significance to it. I do not think it matters one way or another and I mention it only because of the high importance attached to the paper generally.
96. One of the Claimants’ points was that the idea of using β3-AR agonists for treating OAB had “momentum”. I agree with this. Significant results advancing the understanding of the role of β3-ARs in the bladder had been achieved in a period of just a few years leading up to the priority date, and suggestions for therapeutic potential had been made swiftly thereafter. I will need to take account of this when considering β3-AR agonism in the context of the other mechanisms under consideration.
97. The Claimants also rely on the suggestions in the articles mentioned above about future use of β3-AR agonists in human clinical trials for OAB. The way in which the possibility or promise of such clinical trials is expressed varies from author to author, and it is possible to read their statements as being to the effect that the lack of clinical trials was a gap in the art’s knowledge and therefore a limitation, or to the effect that the in vitro and in vivo tests already done were so promising that clinical trials were warranted in short order. One also has to allow for the fact that workers on a particular mechanism will always tend to emphasise potential utility in therapy, and the review articles looked at during the oral evidence contained similar statements for other mechanisms, as well.
98. In this connection, the Claimants relied on the fact that one company in the field, Kissei had just initiated phase 1 clinical trials on a β3-AR agonist at the priority date. The Claimants said that that meant that phase 2 clinical trials were also on the horizon, in the sense that phase 1 trials would not be done without the possibility of phase 2 trials being a real one. They also used the matter as the vehicle for an attack on Dr Mills, who had said that clinical trials of β3-AR agonists for OAB were not yet beginning at the priority date. I hold that the Kissei trials were not CGK in any event (there being no evidence that they were widely known) and therefore so far as the CGK was concerned there was no awareness of clinical trials of β3-AR agonists for OAB. I think the attack on Dr Mills was misplaced, essentially for that reason, although he perhaps should have noted and acknowledged the early Kissei work given that it was identified in a Pfizer review which he put into the case.
99. The increasing understanding of β3-AR agonists in the context of the bladder must also be tempered by the CGK fact, set out in Yamaguchi, that β3-AR agonists had failed in human clinicals trials as anti-obesity agents even after success in animals. One possible reason for this, explained in Yamaguchi, was that the β3-AR agonists tested in the clinic were only weak, partial agonists of the human β3-adrenoreceptor, and not selective for the human β3-adrenoreceptor. More generally, there was a lack of full understanding of the reasons.
100. Overall, I think the CGK was that the lack of clinical trials of β3-AR agonists for OAB was recognised as a gap in the knowledge of the art, that they would probably come soon, and that they had potential, but that their outcome was fairly uncertain.
101. It was also implicit in the Claimants’ position that the skilled team would think that any β3-AR agonist would work to relax detrusor tissue and therefore be likely to work as a therapy for OAB. I do not believe that that was the CGK. Dr McMurray disagreed with the Claimants’ position, and said that it would be expected that not all β3-AR agonists would behave the same, and I accept that evidence. He supported this with evidence, which I also accept, that at Pfizer it was found that some β3-AR agonists which were potent in cell lines did not work well in detrusor muscle, and that predictability for agonists was always difficult and more complex than for antagonists. This work at Pfizer was not, of course, CGK, but it lends reality and support to Dr McMurray’s evidence on this point.
102. Yamaguchi identifies the need to find better β3-AR agonists. What it says about them has an emphasis on selectivity (and that the one that it used, L-755,507, was selective, though no information is given about potency) but clearly also refers to the need for agonists to be full, and potent. Dr McMurray gave an explanation, which I found convincing and accept, that the problem would have been seen to be that the compounds tried had been weak more than that they had been partial agonists. This was the context for a further debate about the CGK situation in relation to the existence of good human β3-AR agonist compounds.
103. L-755,507 is mentioned in Yamaguchi which itself was agreed to be CGK. Other than that there was no evidence that any particular individual β3-AR agonist compound was CGK; the skilled team would think that if they needed one they would try to look up possibilities in the literature. Dr McMurray prepared a table of β3-AR agonist compounds which he gleaned from the papers in the case, the prior art and papers cited in the priority document, the Patent and the prior art. There were a large number and many were both potent and selective. However, only three were shown to be promising β3-AR agonists for the human β3-AR, and one of them was L-755,507 itself.
104. The Claimants argued that no structure for L-755,507 was available (none is given in Yamaguchi); Dr McMurray said that he thought it was. I was not told what source Dr McMurray had in mind, but in view of his general care and reliability I think he is more likely than not to have been correct.
105. Of the other two compounds, one was in a paper by Ok et al., from Merck, and one was from Igawa’s group, referenced in a paper put to Dr McMurray by Counsel for the Claimants, and for which no structure was given in the paper.
106. So the overall picture is that there were many β3-AR agonists, but only a handful of human-selective, potent ones. Two were not CGK, and there was limited CGK information about L-755,507 as I have just explained.
107. The Claimants’ case was that the state of the art in terms of CGK was that clinical trials for OAB were highly desirable and would have been imminent or already underway had it not been for the lack of human-selective, good β3-AR agonist substances. I do not accept that this was the case; the Claimants did not show that the keenness for clinical trials was as strong as they said and they did not demonstrate that there was a general attitude in the field that the one thing holding back the start of clinical trials was the lack of appropriate compounds to test (Dr Argentieri had not said so in his written evidence). Certainly the skilled team would have to go looking for one if they did consider going down that route, but the existence of published work from three significant undertakings using such a compound leads me to conclude that it would not be regarded as an excessive deterrent.
108. In his second report, Dr McMurray presented a “CIR” analysis of the various possible therapies for OAB in the literature. CIR stands for “Confidence in Rationale”. Dr McMurray looked at each possible target mechanism for OAB in the literature and gave it a score based on the evidence in favour of it. He then ranked them. The scheme is rather complicated but essentially gave points for evidence for each mechanism in three bands. I will return to this below. For now I will just say that β3-AR agonism was “mid table”, scoring 5 points out of a possible 15.
109. There are two aspects to the CIR issue on CGK. The first is whether the performance of such an analysis was CGK at all. The second is whether the analysis that Dr McMurray did reflects the CGK about potential therapies for OAB. The Claimants were scathing in their criticisms on both aspects.
110. As to the first aspect, I hold that this kind of analysis was CGK. Dr McMurray said it was well known at Pfizer (which Dr Mills supported), and that he knew at least one other drug company that used it. Dr Argentieri said that in his experience at Wyeth something similar was used, but it was called “SWOT” - “Strength, Weakness, Opportunity, Threat”. Dr Argentieri was praising of the technique and made only minor criticisms of Dr McMurray’s analysis.
111. I hold that CIR was CGK as a structured way to pull together and objectively assess different options for drug discovery and/or development. However, I also hold that it had CGK limitations which I take into account:
i) First, CIR only looks to the rationale for whether a mechanism is likely to provide a therapeutic effect. It does not deal with side effects, which would be dealt with by “CIS” or “Confidence in Safety”, or the ability to deliver the drug (bioavailability, formulation etc.) which would be dealt with by “CIF” or “Confidence in Feasibility”. CIF was not the subject of discussion at the trial before me, but CIS was, and it was clear that Dr McMurray’s analysis was capable of giving a good mark on CIR for a mechanism which, based on the CGK, was likely to be poor or even just unacceptable in terms of side effects.
ii) Second, CIR may come across as more granular or refined than it is. The judgments that it entails do not really allow discrimination between targets which score, say, 5 points and those which score 3 or 4 or 6 or 7. Thus it could be quite sensible to say that β3-AR agonism was “mid-table” but to say that it was definitely less good than a mechanism which scored 6 would not be well-founded.
iii) Third, CIR gives extremely heavy weight to some factors which can give a distorted impression. In particular, any target which was in phase 2 or phase 3 clinical trials or the subject of an approved drug automatically scored 15.
iv) Fourth, CIR was intended to give management at pharmaceutical companies a very rapid way to identify positive and negative factors. It was not intended to deal with nuances.
v) Fifth, CIR as done by Dr McMurray assigned points for types of evidence without regard to strength, recency or topicality. There was nothing to stop a target which had had positive results in the past scoring well even if its progress had been completely stalled for years. This is material because one of the Claimants’ arguments (which I have already said above that I accept) was that β3-AR agonism for OAB had momentum at the priority date, having been the subject of significant advances in the previous few years.
112. Having held that CIR as a technique was CGK, I turn to consider its use in the present case, where it formed a central part, but not the only part, in the argument over the CGK as to other possible therapeutic approaches to the treatment of OAB.
113. There were several streams of evidence about the various possible mechanisms for investigation. In particular:
i) Drs Mills and McMurray co-operated to produce a joint document called “Schedule 1 - Technical Background”. In a section beginning at paragraph 2.22 they listed therapeutic avenues under investigation by way of three categories:
a) Drugs targeting the motor (efferent) neuronal system or by targeting smooth muscle directly;
b) Drugs targeting the sensory (afferent) neuronal system;
c) Drugs targeting the central nervous system (CNS).
And in total there were about 20 options listed.
ii) Dr Argentieri listed 6 approaches in his first report, but with some exceptions these were more specific than the broad categories in Schedule 1:
a) Antimuscarinics;
b) β3-AR agonists;
c) Potassium channels;
d) Nitric Oxide Synthase;
e) Vanilloid receptors;
f) Tachykinin receptor antagonists.
114. As I have touched on above, Dr Argentieri explained in his written evidence introducing these 6 that the first three “were the subject of a lot of interest or recent work in 2002” and he also said that he had worked from his recollection in preparing the list.
115. Although the varying breadth of the headings used mean that a direct numerical comparison cannot be made it is obvious that Dr Argentieri’s list was significantly shorter than Astellas’ experts’.
116. Dr Argentieri said he knew of all the possibilities listed by the Claimants’ experts. He accepted that his omission of P2X1-receptor antagonists was a mistake and that they should have been included. In other respects, he sought to justify leaving things out by means which I thought were not convincing. He said that one reason for leaving things out were if they were of “academic” interest, but the main example he gave as being “academic” was vanilloid receptors, which he in fact included. He said CNS agents were problematic because of the blood/brain barrier, yet they were clearly the subject of significant study, and were included in a review paper which he himself had put forward (Chancellor, 2002 “New Frontiers in the Treatment of Overactive Bladder and Incontinence”).
117. Overall therefore I prefer the evidence of Astellas’ witnesses as to the range of possibilities, which was wide.
118. The detailed effort to assess those possibilities relative to one another was the subject of Dr McMurray’s CIR analysis to which I have already referred. It was contained in exhibit GM4 to his second report.
119. In addition to the general limitations of CIR analysis that I have already discussed above, it should be noted that GM4 was based only on review articles already in the case. Because those had a heavy focus on β3-AR agonism, that approach meant that other materials supporting the use of other mechanisms were not looked for; that could, as Counsel for Astellas pointed out, only favour the Claimants’ position and promote β3-AR agonism relative to other possibilities. Indeed, it is a more general point that the papers in the bundles for trial had a focus on β3-AR agonism that did not reflect its true position in the OAB field, as evidenced by the fact that Dr Argentieri identified 500 OAB papers, from which a selection of those to be put in evidence was made.
120. As I have said, Counsel for the Claimants mounted a strong attack on GM4. The most radical attack was that CIR would just never have been used, and I have already rejected that because it was a CGK technique that was in fact in use in industry. A second layer of attack was that it is not very granular in a number of ways and does not cover safety or feasibility. These are limitations which I have accepted above and I bear them in mind. I think that they only slightly undermine the broad purpose for which Dr McMurray put them forward.
121. The next layer of attack focused on individual points and/or individual mechanisms.
122. An example of an individual point is that Counsel for the Claimants put to Dr McMurray that β3-AR agonists should probably have had one extra point for animal in vitro data. Dr McMurray accepted this, although the force of the point is rather blunted by the fact that quite a lot of the mechanisms have a positive entry for animal in vivo data and none for in vitro, but the latter probably preceded the former, so they all should have one extra point, too. In any event, I do not think Dr McMurray showed any general lack of care, and one extra point is neither here nor there in my assessment.
123. As to individual mechanisms, examples of the criticisms included:
i) Mechanisms working on the CNS would, Counsel for the Claimants said, attract unusually significant concerns about off-target effects. Dr McMurray accepted those matters would have to be considered, but as I have already said, CNS agents were being studied by real teams.
ii) Calcium antagonists were said in two of the review papers not to be supported by available information. I agree with this criticism. Optimism for this mechanism would be very low and it is an illustration of the limitations of CIR.
iii) Agents acting on the dopaminergic pathways were likely to be severely limited by their side effects. Again, I agree with this criticism and it makes the point that CIR only looks at part of the picture.
iv) Vanilloids were unattractive because they would require catherization. But they were clearly being studied and proposed quite a lot, with the goal of e.g. reducing the burning sensation likely to be experienced. So this was not a strong criticism.
v) Prostanoids had not been the subject of any new information for the previous decade. I accept this point and it is another example of one of the limitations of CIR to which I have already referred.
124. I thought that these more specific attacks on the CIR analysis made some real progress; some of the options put forward would have been very likely to be rejected by the skilled team for one of the reasons identified above and/or to be considered significantly less attractive than β3-AR agonists. I also thought that the 15 “automatic” points given to improved antimuscarinics and M3 receptors gave an unbalanced picture, as Counsel for the Claimants submitted, because it just meant that the established treatments worked. The relevance of that to the obviousness or otherwise of a potential new approach is low.
125. However, I thought the overall impact of the CIR analysis was similar to the impression that I gained from the narrative evidence and from the cross-examination of Dr Argentieri, which was that this was a field where there was known to be a real problem with the existing treatments, and in which there were a significant number of possibilities to be considered, none of which was the clear favourite, and none of which had an overwhelmingly clear rationale or body of evidence. The lack of a clear direction forward was, in a way, evidenced by the willingness in the field to press on with approaches like vanilloids with their obvious apparent challenges. This fits with my view that there was no established field of β3-AR agonists and that drug companies in the field were typically trying multiple approaches. Some but not all of the active research programmes included β3-AR agonists, and some who started work on β3-AR agonists later gave up on it, for example Wyeth (because of safety concerns specific to its business and therefore of no great relevance for me) and Pfizer in about 2003 for what it regarded as insufficient CIR.
126. After some basic explanation about the shortcomings of existing anticholinergic treatments for OAB and the rising numbers of sufferers (at [0001]-[0002]), the Patent identifies at [0004] that in the international application `607 that gave rise to `288 and `111, mirabegron was reported to be useful for promoting insulin secretion, enhancing insulin sensitivity, and for anti-obesity and anti-hyperlipemic activity. It points out, however, that the application did not disclose use for treating overactive bladder.
127. Then at [0005] the specification refers to another patent application, WO/98/07445, which is said to be relevant to bladder conditions, and which mentions the compound CGP-12,177A. As will appear below, this compound is used as a comparator in the experimental work in the Patent; it is a partial β3-AR agonist.
128. Some more detail about mirabegron and about OAB is given in [0013] at the start of the “Disclosure of the invention section”. This explains that the inventors’ identification of mirabegron arose from their work on its use for diabetes. There was some cross-examination about this paragraph but I did not think it led anywhere.
129. There follows some detailed description about synthesis which is not relevant to my task. It stretches to [0026].
130. From [0027] to [0042] three experimental examples are set out (there is a fourth, concerning formulation, from [0043] to [0044] but it is irrelevant).
131. Example 1 is done in an in vitro model using strips of rat detrusor muscle which are made to contract by the application of carbachol and of potassium chloride. The relaxant effect of mirabegron is assessed and compared with that of CGP-12,177A. Mirabegron is seen to achieve much greater relaxation and at lower concentrations; this is expressed in a variety of ways but the details do not matter.
132. Example 2 is an in vivo model in rats. Rhythmic bladder contraction was experimentally induced in the anesthetised animals and frequency and pressure of contractions was measured for different concentrations of mirabegron. Vehicle was used as a control.
133. Dose dependent reduction in contraction frequency was seen for mirabegron but not for the control (Fig 3) and this is explained to show clinical utility for overactive bladder (“is believed to be clinically effective …”).
134. Contraction pressure was not affected until the highest dose was given (Fig 4) and the specification explains that that would be preferred because it indicates that urine retention is not induced.
135. Example 3 is also an in vivo rat experiment. Overactive bladder was chemically induced and saline injected into the bladder to induce a micturition reflex. The average interval for urination was measured before and after administration of mirabegron and was longer after administration (Fig 5). The specification again says that this indicates clinical efficacy for overactive bladder (“is believed to be …”).
136. [0042] summarises what has been done and the implications for utility:
[0042] Thus, the active ingredient of the present invention shows a strong bladder relaxation action in "isolated rat bladder smooth muscle relaxation test", decreases the contraction frequency of rhythmic bladder contraction on a dose-depending manner in "rat rhythmic bladder contraction measurement test" and prolongs the micturition interval in "micturition function measurement test on cyclophosphamide-induced overactive bladder model rat" whereby it is clinically useful as a remedy for overactive bladder. In addition to overactive bladder as a result of benign prostatic hyperplasia, it is also able to be used as a remedy for overactive bladder accompanied with urinary urgency, urinary incontinence and pollakiuria.
137. It should be borne in mind that all this work was done in rat-based models; there is no work relating to humans. There is also no test of whether mirabegron is selective for β3 in preference to β1 or β2.
138. Claim 1 of the Patent is as follows (I replace the chemical name with “mirabegron”):
“A remedy for use in the treatment of overactive bladder comprising [mirabegron] or a salt thereof as an active ingredient.”
139. This is of course a second medical use claim. Counsel for Astellas pointed out that unlike many second medical use claims, no first medical use was established, in the sense of a successful or approved drug. This is true and I have taken account of it, but it does not affect the meaning or scope of the claim.
140. Other dependent claims are more specific in relation to the conditions to be treated, in particular claim 5 for urinary incontinence and claim 6 for pollakiuria, but they were relevant only to the priority issues and for the purposes of this judgment all that matters is claim 1.
141. `288 was published on 6 May 1999, was filed on 13 October 1998 and claims a priority date of 17 October 1997. The applicant was Yamanouchi Pharmaceutical Co, Ltd., of which Astellas is the successor.
142. The title of `288 is “Amide derivatives or salts thereof”. The abstract refers to “an amide derivative” according to a Markush formula there set out. It goes on to say:
A therapeutic agent for diabetes mellitus having both an insulin secretion promoting action and an insulin sensitivity potentiating action and also having anti-obesity and anti-hyperlipemia actions due to a selective stimulating action to β3-receptors, is also disclosed.
and the “Technical Field” is described on page 1 as relating to novel compounds and treatments for diabetes mellitus.
143. The Background Art section starts on page 1 and its first three paragraphs discuss diabetes and its therapy in general terms. The last paragraph on page 2 says this:
U.S. Patents 4,396,627 and 4,478,849 describe phenyl-ethanolamine derivatives and disclose that those compounds are useful as drugs for obesity and for hyperglycemia. Action of those compounds is reported to be due to a stimulating action to β3-receptors. Incidentally, it has been known that b-adrenaline receptors are classified into β1, β2 and β3 subtypes, that stimulation of β1-receptor causes an increase in heart rate, that stimulation of β2-receptor stimulates decomposition of glycogen in muscles, whereby synthesis of glycogen is inhibited, causing an action such as muscular tremor, and that stimulation of β3-receptor shows an anti-obesity and an anti-hyperglycemia action (such as decrease in triglyceride, decrease in cholesterol and increase in HDL-cholesterol).
144. That leads into and provides context for the first paragraph on page 3 which was touched on in the evidence:
Unfortunately, those β3-agonists also have actions caused by stimulation of β1- and β2-receptors such as increase in heart rate and muscular tremor, and they have a problem in terms of side effects.
145. There follows an explanation that compounds which are selective in rodents may not be so in humans, and that β3-AR agonists which are selective in humans have become a subject of interest. An example is given; the explanation is still concerned with obesity, hyperglycemia, insulin secretion and insulin sensitivity.
146. The Disclosure of the Invention begins on page 4. The first sentence of it was the focus of oral argument and cross-examination, although it is in very similar terms to what has gone before:
The present inventors have conducted an intensive investigation on compounds having both an insulin secretion promoting action and an insulin sensitivity potentiating action and found that novel amide derivatives show both a good insulin secretion promoting action and a good insulin sensitivity potentiating action and furthermore show a selective stimulating action to β3-receptors, leading to accomplishment of the present invention.
And over the page at the top of page 5 there is some more reference to anti-obesity and anti-hyperlipemia actions, and treatment for diabetes.
147. There is then a long section about synthesis irrelevant to this trial, which goes on until page 15, where a section entitled “Industrial Applicability” begins. The first paragraph of the section is repetitive of earlier statements and I need not quote it, but there follows an important passage, which touches on the experimental work that is to follow, on the role of β3-AR agonism on what has been found, and aspects of hoped-for clinical utility:
As confirmed by a glucose tolerance test and a hypoglycemic test in insulin-resisting model animals as described later, the compound of the present invention has both a good insulin secretion promoting action and a good insulin sensitivity potentiating action, so that its usefulness in diabetes mellitus is greatly expected. Although the β3-receptor stimulating action may have a possibility of participating in expression of the insulin secretion promoting action and the insulin sensitivity potentiating action, other mechanism might also possibly participate therein, and the details thereof have been still unknown yet. The β3-receptor stimulating action of the compound of the present invention is selective to β3-receptors in human being. It has been known that the stimulation of β3-receptor stimulates decomposition of fat (decomposition of the fat tissue triglyceride into glycerol and free fatty acid), whereby a disappearance of fat mass is promoted. Therefore, the compound of the present invention has an anti-obesity action and an anti-hyperlipemia action (such as triglyceride lowering action, cholesterol lowering action and HDT cholesterol increasing action) and is useful as a preventive and therapeutic agent for obesity and hyperlipemia (such as hypertriglyceridemia, hyper-cholesterolemia and hypo-HD-lipoproteinemia). Those diseases have been known as animus factors in diabetes mellitus, and amelioration of those diseases is useful for prevention and therapy of diabetes mellitus as well.
148. This contains some expansion of the information given earlier. Counsel for Astellas also relied on the fact that it says that although β3-AR agonism may have a possibility of participating, other mechanisms might as well, and the details were not yet known; he emphasised the uncertainty around even the conditions that `288 focuses on. Counsel for the Claimants responded that the skilled addressee would think there were specific reasons for the uncertainty arising from insulin producing cells not having β3-receptors and so it would not detract from the teaching that the compounds were β3-AR agonists. I did not find this convincing; `288 is expressing doubts even in relation to that which it specifically concerns, and it is not natural just to shrug them off when thinking of applying the teaching in a different setting.
149. On page 17 there is a further list of conditions that selective β3-AR agonism might prevent or treat:
Further, the selective β3-receptor stimulating action of the compound of the present invention is useful for prevention and therapy of several diseases which have been reported to be improved by the stimulation of β3-receptor. Examples of those diseases are shown as follows.
It has been mentioned that the β3-receptor mediates the motility of non-sphincteral smooth muscle contraction, and because it is believed that the selective β3-receptor stimulating action assists the pharmacological control of intestinal motility without being accompanied by cardiovascular action, the compound of the present invention has a possibility of being useful in therapy of the diseases caused by abnormal intestinal motility such as various gastrointestinal diseases including irritable colon syndrome. It is also useful as the therapy for peptic ulcer, esophagitis, gastritis and duodenitis (including that induced by Helicobacter pylori), enterelcosis (such as inflammatory intestinal diseases, ulcerative colitis, clonal disease and proctitis).
150. This list, which Counsel for Astellas called the “laundry list” does not mention OAB. A possible reason is that the work looking at the presence and function of β3-receptors in the bladder that I refer to above in dealing with the CGK had not yet been done, or reported.
151. There follow two further short paragraphs which raise the additional possible applications of neurogenic inflammation and depression.
152. `288 then moves on to experimental work. At page 18, second full paragraph, the authors say:
The action of the compound of the present invention has been ascertained to be selective to β3-receptors as a result of experiments using human cells, and the adverse action caused by other β3-receptor stimulation is low or none.
153. The last reference to “β3-receptor stimulation” is a typo and should just be to “β-receptor” as is obvious from the context and as Dr McMurray had pointed out in his written evidence.
154. This paragraph has an ambiguous turn of phrase which appears elsewhere in the document: the singular form “the compound of the present invention” does not make it clear whether just a single compound had its “action … ascertained”, or more than one. It cannot very well mean any compound of the invention since clearly not all within the Markush group could have been tested.
155. Three different experiments are then described.
156. The first is a hypoglycemic test in mice. I do not think the experimental details matter, save that the metric was reduction in blood sugar level. The outcome is reported in the first full paragraph on page 19:
The compound of the present invention significantly lowered the blood sugar level as compared with that prior to the administration of a comparative drug in both cases of oral and subcutaneous administrations. For example, the compound of Example 6 showed a hypoglycemic rate of 48% in average by oral administration of 10 mg/kg. From this result, it is shown that the compound of the present invention has a good potentiating action to insulin sensitivity.
157. This is the only concrete, numerical data in `288. It concerns Example 6, which is not mirabegron, as I explain below.
158. The second experiment is another animal test, this time in rats. Again the details do not matter, but what was observed was an increase in insulin levels and an inhibition of blood sugar increase in animals receiving “the compound of the present invention”. This time, no numerical data is given.
159. The third experiment is done in cell lines expressing human β-receptors. Two different cell lines were used; the details do not matter. What was tested for was the stimulating effect on cAMP production. The authors say at the foot of page 20 that “Intensity of action of each compound was compared …” so in this instance it appears that more than one compound was tested. Over on page 21 there is the statement that “It has been ascertained that the compound of the present invention has a selective stimulating action to human β3-receptor”. There is no numerical data. This is the result that Dr Argentieri called “compelling”, which in my view, given the context, can be seen to be a significant overstatement.
160. It was accepted by Dr McMurray that the test methods used were standard and I did not detect any dispute about them.
161. In a section entitled “Best Modes for Conducting the Invention” starting on page 23, six examples of compounds with some details of their synthesis are given. The Best Modes section begins with the following introduction, to which Counsel for the Claimants referred in opening and which was touched on in the oral evidence by both sides:
The present invention is further illustrated by way of Examples as hereunder. Compounds of the present invention are not limited to those mentioned in the following Examples but covers all of the compounds represented by the above formula (I), salts thereof, hydrates thereof, geometric and optical isomers thereof and polymorphic forms thereof.
162. The structural formulae for the six examples are given in Table 1. Example 5 is mirabegron. Example 6 is the one for which numerical data is given in the hypoglycemic mouse test that I have mentioned above.
163. Some of the six compounds are structurally similar to each other and some are not. Dr Argentieri accepted that Examples 1, 4 and 6 were similar one to another while 2, 3 and 5 stood out as different, and he accepted that even for the (relatively) similar ones it was not possible to infer from their structure that they would have the same activity.
164. The Claimants argued that `288 teaches that all six examples were tested for β3 selectivity. On the other hand, Astellas argued that apart from the test on Example 6 in the mouse hypoglycemia test, which it said was irrelevant to OAB, it was not clear which other tests were done on which, if any, compounds.
165. In general, Astellas is clearly right about this. There is nothing to say which compounds were tested in which experiment and the use of the singular form “the compound” is unhelpful to the Claimants.
166. The high point of the Claimants’ argument is, however, the reference at the bottom of page 20 to “Intensity of action of each compound was compared …” in its context. Counsel for the Claimants made some progress with Dr Mills on this, taken together with the “Best Modes” text on page 23, but it was on the basis that the text at page 20 actually said that the six were tested, and the text on page 23 confuses the picture because it seems to say that all compounds contemplated by the document including all those within the Markush group of formula (I) are “compounds of the invention”.
167. What the text on page 20 means is ultimately a matter for me and not the experts. The reality is that it is not at all clear. Leaving aside the semantic picking apart of “the compound” and “each compound”, it is striking that in just one instance actual numerical data is given for a compound, Example 6, and even that not in the selectivity assay. Why would the authors include that and then be so vague about describing what they had done in the third, selectivity test, if they had in fact tested all six Examples with success? Why would they not include numerical data? My overall conclusion is that the skilled addressee would think that no safe conclusion could be reached over what testing had been done other than the one data point for Example 6. That does not mean that they would think the teaching could not usefully be progressed; they would have the hope that if they tested the six Examples they might get some positive results, but they would have no expectation for any particular compound, other perhaps than for Example 6 where it might be a bit more likely that selectivity had been tested, but from which no conclusion about other compounds could be drawn without testing.
168. The argument over which compounds were tested for selectivity rather overshadowed a related point which I think is of importance, which is that on any view there is no data about the affinity or potency of any of the compounds and no efficacy test of any relevance to OAB.
169. Before leaving `288’s disclosure, I should deal with a point made by the Claimants against Dr Mills, who had said that there was nothing of interest to the skilled clinician in `288. In cross-examination Dr Mills said that the clinician would have no interest in the compounds disclosed because there was no clinical data relevant to OAB. I did not find this surprising and Prof Abrams had said much the same thing. I do not think that it means that the document would be of no interest to the skilled team, only that assessment of its teaching would be in the province of the skilled pharmacologist so that the evidence that matters is that of Dr Argentieri and Dr McMurray.
170. I will use the Pozzoli analysis.
171. I have identified the skilled team and the CGK above.
172. `288 identifies mirabegron among other compounds. The steps to the claims of the Patent are the choice of mirabegron and its use to treat OAB as opposed to the conditions mentioned in `288.
173. This is the real area of contention.
174. The nature of the Claimants’ case is that:
i) It was CGK that selective β3-AR agonists had the potential to treat OAB.
ii) There was a shortage of potent human, selective β3-AR agonists.
iii) Therefore when in that context the skilled team saw some selective β3-agonists in `288 they would be of interest.
iv) It would therefore be obvious to test the 6 compounds in `288 in a detrusor strip assay with the expectation that they would induce relaxation.
v) It would be obvious thereafter to take those that succeeded, or at least mirabegron, into clinical trials with a reasonable expectation of success.
175. This is my paraphrase. In their opening written submissions the Claimants put it even more simply (paragraph 8):
The Claimants’ case is straightforward. They submit that by the priority date it was part of the common general knowledge that β3-agonists had the potential to be used to treat OAB and that consequently it was obvious that compounds disclosed as β3-adrenoceptor agonists were potential therapeutics. Mirabegron had been disclosed in the AU288 Application as a β3-adrenoceptor agonist and it follows that no technical contribution resides in identifying that it has potential for use in treating OAB.
176. The Claimants’ case is a little unusual in that the CGK does nearly all the heavy lifting and provides all the rationale and motivation; the function of the cited prior art is simply to provide the identification of mirabegron to be plugged into an argument which could apply to any selective β3-AR agonist.
177. Astellas’ key responses are that:
i) β3-AR agonism was just one of a number of possible ways of treating OAB under consideration by the art.
ii) There was no clinical evidence yet that β3-AR agonism would work to treat OAB.
iii) β3-AR agonism had been unsuccessful in the obesity field.
iv) `288 is not about OAB at all and does not even mention it.
v) `288 gives no information about mirabegron’s activity.
vi) `288 does not improve the CIR for β3-AR agonism.
vii) If β3-AR agonism were to be pursued there were many more attractive compounds to choose from than mirabegron.
viii) Although it accepted that it cannot rely on concerns over possible side effects in general because of the fact that the Patent contains no information about selectivity, there would have been a concern about urine retention, which would not be a side effect arising from lack of selectivity, but rather from β3-AR agonism itself. That, Astellas says, is addressed by the Patent.
178. I will deal with the retention point separately below. I reject point vi) as being of any real significance, since it was not the Claimants’ case that `288 improved the CIR for β3-AR agonism or contributed to it at all. The Claimants’ case was that adequate CIR came from the CGK, as exemplified by Yamaguchi in particular.
179. Each side relied on what it said was key cross-examination in which a really telling blow had been struck. Such “soundbites” can be very important or even decisive but it is always important to look at them carefully and in context. That is what I have tried to do, and I have re-read these key passages, their context, and other related passages. The fact that I have quoted just a couple does not mean that they are the only ones I have considered.
180. The Claimants relied strongly on the following from Dr Mills (T3/326-327):
Q. Even before you read the priority document you would have expected a ß3-agonist to be effective to treat overactive bladder, would you not, even before reading the priority document?
A. You would expect that it would have potential to treat overactive bladder.
181. And the following from Dr McMurray (T4/375):
Q. Just going back to 2002, because obviously all that came later, certainly the common general knowledge of the skilled person, looking at Yamaguchi, would be that if you got a ß3-agonist you would expect it to have an impact on a rat detrusor away [sic, sc. "assay"]?
A. I think the common general knowledge would be as a pharmacologist who had worked in GPCRs and understood agonism, you would say that if you got a good potent, good affinity compound, it is likely that you would see an effect. That does not necessarily translate for all compounds by any means whatsoever.
182. These are to similar effect - indeed it might be said that they are duplicative - and I think, and thought at the time, that both witnesses were careful about how they expressed themselves. They both made clear that it was possible there would be an effect, but that there might not be. Dr Mills did so by saying that there was a potential, and Dr McMurray said that an effect was likely for a good potent, good affinity compound, but that that did not translate for all compounds (and it was clear from the rest of his evidence that he did not accept that mirabegron was shown in the prior art to be a good compound).
183. The context is also important: on the next page from that quoted above, Dr Mills said such a compound “could” have a treatment effect, and that evidence of the kind put to him was “consistent” with it. Dr McMurray’s evidence continued (I have touched on this in dealing with the CGK):
Q. It sounds to me like you were a bit surprised that when you went to Pfizer, actually you were seeing compounds that did not work?
A. No, again as a GPCR pharmacologist it was very common. The whole concept of partiality and agonism, it is always much easier to work with antagonists than it is to work with agonists. The reason for that is that the only important measure with an antagonist is affinity. That is its capability of binding to the site. Once it gets there, it just stops everything else. With an agonist you have both affinity and then you have the amount of coupling within the system. It is going to interpret your binding and your little bit of activation into an effect. That just, you know, becomes immeasurably more complex, whenever you are dealing with an agonist, than if you are just dealing with an antagonist.
184. So he gave further reasons to explain why it was too simplistic to assume that any β3-AR agonist would work.
185. For its part, Astellas relied on passages from the cross-examination of Dr Argentieri which they said reflected a false approach to obviousness. In particular, they relied on the following, T2/189-192:
Q. I do not know whether you have a boss somewhere in the company, I presume you do, you went along to your boss and you said, “Look, I have found a patent that tells me there are these six compounds which they think are b3-agonists. I do not know what their potency is. I do not know what their specificity is. They seem to have tried them for diabetes. They think they are good for diabetes. They do not give me any data. Nevertheless, I want to select these six over all the other b3-compounds that are in the literature, including b3-compounds that have quite promising data, actual data, including one that Wyeth, themselves, made. I am going to ignore all those ones, I am going to test these six”. Are you seriously saying that your boss would say, “Yes, what a good idea, I will give you the money for that”?
A. So the question that was put before me was given this patent, forget everybody else's patents, forget everybody else's molecules, given this patent would it be unreasonable to synthesise all six compounds and test them? And my answer to that is no.
Q. So you were told to ignore any other compounds that had been discussed in the prior art?
A. I was told to consider this patent only.
Q. And you were not asked to do it on the basis of the data relating to these compounds, compared to data relating to any other compounds ----
A. Correct.
Q. ---- because you were told to ignore other compounds?
A. Correct.
Q. And you were not asked whether you thought there was a good chance of developing something out of these six?
A. They asked my opinion, and I think my opinion was that, in 1997, given what is taught in this patent, that there was quite a good chance that something useful for OAB would have come out of this.
Q. Really? Although this does not mention OAB at all?
A. That is correct.
Q. Even in what I have called the kitchen sink list?
A. And here is an example getting ahead of the competition; right? So if you want to wait till everything is laid out for you all nice and tidy, then every other company with an interest in OAB is going to jump at it. And so the skilled pharmacologist has to be a little crafty here, and try and dig through assumptions and interpret statements that will help him become competitive. And, again, what we knew at this time was that the b3-receptor was an attractive target for OAB. And here are compounds which are being described as selective b-receptors, and not only selective b3-receptors, but human b3-receptors, which, as you recall, was a big deal back then, to distinguish between the rodent and the human. A lot of the compounds that had preceded this did not, they were active at the rodent receptor and not the human receptor, so these were special compounds.
Q. What you are describing the skilled team as doing is rather akin to the punter at the race course who decides “I am not going to go for the favourite, I am not going to go for the second favourite, I am going to go for one of the outsiders. I am going to put my bet down on something which no one else has considered to be a good prospect”; is that not right?
A. Not that other people did not consider it was a good prospect, that you considered it was a good prospect, and therefore it gives you a competitive advantage. That is very important. “I am smarter than everybody else, I am going to take advantage of this”.
186. Astellas pointed especially to what it said was a direction to Dr Argentieri to ignore all other compounds, and to his conception of the skilled person being smarter than everyone else. The Claimants’ response was that it was not put that the witness had not given his evidence from the perspective of the ordinary skilled addressee. I disagree. Taken with Dr Argentieri’s evidence as a whole I think he was effectively challenged and given an opportunity to answer, and did have in mind a more crafty and insightful frame of mind than that possessed by the notional uninventive addressee. I also think he had in mind a narrow and incorrect question of whether it was, divorced from the CGK, unreasonable to make the compounds of `288. That is not to say that he was without technical grounds for his opinions, but his focus was not right.
187. As I have mentioned above, Astellas sought to argue that the skilled team would be deterred from trying mirabegron (and β3-AR agonists generally) by a concern that the very action of relaxing the detrusor would lead to incomplete voiding of the bladder. Astellas said that as distinct from potential side effects arising from a lack of selectivity, this concern was addressed by the Patent in [0036].
188. I agree that Example 2 as reported in [0036] does contain information that the skilled team would find reassuring in relation to retention, so in principle this point was open to Astellas as a possible problem that is addressed by the Patent. I have to say that it was not flagged very clearly until late on, however.
189. In any event, the point fails on the facts because this concern had been addressed by workers in the field, and that was part of the CGK. A number of references that I was taken to by Counsel for the Claimants in oral closings made this clear, including the references 15 and 18 in the Yamaguchi paper, which itself in the left hand column of page 27 refers to the former paper showing an increase in bladder volume with no change of micturition pressure or threshold pressure.
190. Dr Mills could not point to any paper expressing this concern, and the cross-examination of Dr Argentieri made no progress, it merely being established that in one of the papers of which he was an author (Woods et al, 2001, Efficacy of the β3-adrenergic receptor agonist CL-316243 on experimental bladder hyperreflexia and detrusor instability in the rat) there was a very small effect on voiding. I accept his evidence that the effect was only at higher doses, well above the level where a (very good) effect on bladder instability was seen.
191. I therefore reject this point. That does not positively mean that the Patent is obvious, but this line of defence by Astellas cannot help it.
192. Although there are important arguments in both directions, I have come to the conclusion that the obviousness attack over `288 fails. I will explain my reasons, without I hope unduly repeating what I have already said.
193. It is true that at the priority date the β3-AR agonism mechanism had “momentum” relevant to OAB arising from the recent advancements in understanding that I have identified in relation to the CGK, and hence β3-AR agonists had, in a general sense, potential as agents to treat OAB.
194. However, the Claimants’ case suffers from the two defects of overstating the confidence that that would give the skilled addressee, and of oversimplifying the situation, in particular to the effect that any β3-AR agonist would be likely to succeed as a treatment. One can see these two problems clearly in the formulation of the Claimants’ case that they put forward in their written opening and which I have quoted above.
195. As to overstating the skilled addressee’s confidence in β3-AR agonism for OAB, I bear in mind that the mechanism had not been used successfully in any drug for any condition, and it had failed for diabetes. While there were potential reasons for this that would leave open the possibility of success for OAB, this is not a good starting point for the Claimants.
196. As to the clinical prospects for OAB, the review papers, examples of which I have given above, certainly said that clinical trials were needed for β3-AR agonists for the condition, but my overall assessment of those, as I have touched on already, is that the authors were saying that clinical evidence was what was missing and should be looked for, not that it would necessarily fall into place. Doing those clinical trials would be an exercise in hoping to find something new and promising, not a routine matter with a strong or clear expectation of positive results.
197. I also think the appropriate caution as it would be seen by the skilled addressee is evidenced by the large number of possibilities in play to improve the existing treatments for OAB. Some companies were exploring β3-AR agonism (along with other mechanisms) but others were not.
198. So I thought that Dr Mills and Dr McMurray gave a much fairer impression of the state of play in seeking to improve OAB treatments than did Dr Argentieri: it was possible that β3-AR agonists would work for OAB and it was possible that they would not. The same could be said for a number of the other mechanisms under consideration for OAB that I have touched on above.
199. As to oversimplification, the central problem facing the Claimants seemed to me to be the poor quality of the disclosure of `288 as it applied to mirabegron in particular and the Examples generally, with the very limited data given. It was because of that that the Claimants had to contend, effectively, that any selective β3-AR agonist would be seen as obvious to use for the treatment of OAB. My findings on the evidence as set out above are that that is not so and was not the perception of the skilled addressee. It could not be assumed that any β3-AR agonist would work and it could not be predicted that the results for one would necessarily apply to another. The Claimants put to Astellas’ witnesses, and argued, that no β3-AR agonist had ever failed to show activity in detrusor tissue, but I accept the answer given, that failures would not be published, and I have already said that Dr McMurray gave evidence that at Pfizer some agonists found to be potent in cell assays did not work in detrusor muscle.
200. This does not mean that the skilled addressee would positively think that mirabegron or the other Examples in `288 would not work, but it does mean that there would be a substantial degree of uncertainty. Furthermore, `288 does not “show its working”; the choice of compounds and structures to explore and test is not explained. The reader would probably expect that the thinking was shaped by the application that the authors had in mind (diabetes), and I was not at all convinced by the Claimants’ response to that, which was that it did not matter what condition the authors were working on, provided that they came up with β3-AR agonists in the end.
201. The Claimants tried to bring some unity and reality to their arguments about β3-AR agonism on the one hand and `288 on the other by the contention that the mechanism had been seen as extremely attractive for some time by the priority date, but was held up by the lack of appropriate compounds. Then, it was said, `288 would provide a good way forward for the first time. I have rejected this on the facts in dealing with the CGK. At least some other suitable compounds were around, and the skilled addressee would not think that there was a limitation such that they would naturally decide to proceed with the ill-characterised compounds in `288. As I have said, the argument was also unconvincing because Dr Argentieri had not with any clarity spelled it out in his written evidence, and I accept Astellas’ contention that it only really surfaced in the Claimants’ opening oral submissions.
202. It is of some relevance that `288 does not mention OAB in the list of possible conditions to be treated, but I do not think that it is a critical point in isolation, mainly because of my view that there is force in the Claimants’ argument that the skilled addressee would think OAB’s omission might be explained by `288 having been written before the advances I have identified above. In the course of oral closing argument and in the context of this point, I asked Counsel for Astellas whether he would accept that if `288 disclosed mirabegron as an excellent, potent and specific β3-AR agonist, it would then be obvious to try it for OAB, despite the condition’s not being mentioned in the list. Initially he accepted that it “probably” would be (although he caveated this shortly afterwards) and Counsel for the Claimants understandably sought to leverage this so as to argue that all the Claimants had to show was that `288 in fact did disclose mirabegron as a good compound (in being selective). I certainly did not think that Counsel for Astellas was abandoning all its points about the general uncertainty around β3-AR agonism and OAB, and in my view the shortcomings of `288 and the art’s perception of β3-AR agonism have to be seen and assessed as a whole.
203. Finally, a point made by the Claimants was that the effort involved in making the six compounds exemplified in `288 would not be great. I accept that so far as it goes, and Dr McMurray did accept that the effort in making 23 compounds in the context of a commercial research organisation would not be “unreasonable”, but it is a small part of the picture and one still has to inquire which 6 compounds (or 23) to make, for what purpose and with what confidence that they might succeed.
204. The Claimants’ written opening did not mention insufficiency as a separate attack at all. The last two paragraphs of their written closing submissions briefly made the contention that the Patent “is no more enabling than the prior art”, which was one of the pleaded grounds. However, the argument at trial was not about enablement on either side (possibly it was more live when the issues were more numerous). The Claimants went on in those paragraphs to say that the Patent does not include data from clinical trials or in human tissue, or as to selectivity. While factually correct, I do not think those are really points about enablement at all (they were Conor-type points, which I have addressed above in dealing with the law), and Counsel for the Claimants did not devote any time to insufficiency in oral closing submissions.
205. In any event, the disclosure of the Patent is quite different from that of `288. It focuses in specifically on mirabegron, teaches its use in treating OAB, and gives specific, concrete results in identified assays, albeit not in humans or human tissue.
206. I reject the insufficiency allegation.
207. I conclude that:
i) The obviousness attack over `288 fails.
ii) The insufficiency squeeze fails.
iii) Accordingly, the Patent is valid.
iv) The Patent would be infringed by the Claimants’ proposed acts, their having accepted that there would be infringement if the Patent were valid.
208. I will hear Counsel as to the form of Order if it cannot be agreed. I direct that time for seeking permission to appeal shall not run until after the hearing on the form of Order (or the making of such Order if it is agreed). I draw attention to paragraph 19.1 of the Patents Court Guide, which says that a hearing on the form of Order should take place within 28 days of hand down, which in this case means by 30 June 2022. I ask the parties please to liaise straight away to find a suitable time within that period. If there are any difficulties with this they should be communicated via my clerk promptly.









