S.I. No. 63/1953 -- Standard Specification (Edible Gelatine) Order, 1953.
|
S.I. No. 63/1953: STANDARD SPECIFICATION (EDIBLE GELATINE) ORDER, 1953. |
||||||||||||||
|
STANDARD SPECIFICATION (EDIBLE GELATINE) ORDER, 1953. |
||||||||||||||
|
I, SEAN F. LEMASS, Minister for Industry and Commerce, in exercise of the power conferred on me by subsection (3) of section 20 of the Industrial Research and Standards Act, 1946 (No. 25 of 1946), hereby order as follows :-- |
||||||||||||||
|
1. This Order may be cited as the Standard Specification (Edible Gelatine) Order, 1953. |
||||||||||||||
|
2.--(1) The specification set forth in Part II of the Schedule to this Order is hereby declared to be the standard specification for the commodity described in Part I of the said Schedule. |
||||||||||||||
|
(2) The said standard specification may be cited as Irish Standard 37: 1953. |
||||||||||||||
|
SCHEDULE. |
||||||||||||||
|
PART I. |
||||||||||||||
|
EDIBLE GELATINE |
||||||||||||||
|
PART II. |
||||||||||||||
|
SPECIFICATION |
||||||||||||||
|
In this specification, the letters I.S., when followed by two sets of numbers, refer to the Irish Standard of which the first is the serial number and the second the year of its promulgation by the Minister for Industry and Commerce. |
||||||||||||||
|
In this specification, the letters B.S., when followed by two sets of numbers, refer to the British Standard of which the first is the serial number and the second is the year of its publication by the British Standards Institution. |
||||||||||||||
|
SCOPE |
||||||||||||||
|
1. This specification covers the requirements for edible gelatine, |
||||||||||||||
|
DEFINITION |
||||||||||||||
|
2. Gelatine shall consist of clean wholesome protein obtained by extraction from collagenous material. |
||||||||||||||
|
FORM |
||||||||||||||
|
3. Gelatine shall be powdered, flaked, leaf, kibbled, granulated, or in the form of cake or sheets. |
||||||||||||||
|
ODOUR AND TASTE |
||||||||||||||
|
4. A five per cent. solution of the gelatine at a temperature of 60°C. shall be free from objectionable taste and offensive odour. |
||||||||||||||
|
MOISTURE CONTENT |
||||||||||||||
|
5. The moisture content, when determined by the method described in Appendix B, shall be not more than 16 per cent. |
||||||||||||||
|
pH VALUE |
||||||||||||||
|
6. The pH value of the gelatine, when determined by the method described in Appendix C, shall be not more than 7·0 and not less than 4·0. |
||||||||||||||
|
FREEDOM FROM IMPURITIES |
||||||||||||||
|
7. The gelatine shall be free from all foreign matter and chemical preservatives other than sulphur dioxide. |
||||||||||||||
|
CONTENT OF SULPHUR DIOXIDE |
||||||||||||||
|
8. The content of sulphur dioxide, when determined in the manner described in Appendix D, shall be not less than 400 and not more than 1,000 parts per million. |
||||||||||||||
|
CONTENT OF ASH |
||||||||||||||
|
9. The gelatine, when tested by the method described in Appendix E, shall contain not more than 3·25 per cent. by weight of ash. |
||||||||||||||
|
CONTENT OF ARSENIC |
||||||||||||||
|
10. The content of arsenic, expressed as As, when determined by the method described in Appendix F, shall be not greater than 2·0 parts per million. |
||||||||||||||
|
CONTENT OF HEAVY METALS |
||||||||||||||
|
11. The content of lead, copper or zinc, when determined in the manner described in Appendix G, shall be not more than |
||||||||||||||
|
||||||||||||||
|
TOTAL BACTERIOLOGICAL COUNT |
||||||||||||||
|
12. The total bacteriological count, when determined by the method described in Appendix H, shall be not greater than 10,000 per gram. |
||||||||||||||
|
JELLY STRENGTH |
||||||||||||||
|
13. The Bloom jelly strength, when determined in the manner described in Appendix J, shall conform to the value agreed to by the purchaser and vendor. |
||||||||||||||
|
Alternatively the jelly strength, when tested in the manner described in Appendix K, shall be not less than the jelly strength of the agreed sample. |
||||||||||||||
|
SOLUBILITY OF PARTIALLY SWOLLEN SHEET |
||||||||||||||
|
14. The solubility of a partially swollen sheet, when determined by the method described in Appendix L, shall be not less than that of an agreed sample. |
||||||||||||||
|
MELTING POINT |
||||||||||||||
|
15. The melting point of the gelatine, when determined by the method described in Appendix M, shall be within ± ½°C. of that of an agreed sample. |
||||||||||||||
|
WATER ABSORBTION |
||||||||||||||
|
16. The water absorption of the gelatine, when determined by the method described in Appendix P, shall be within ± 5 per cent. of that of an agreed sample. |
||||||||||||||
|
COLOUR |
||||||||||||||
|
17. The colour of the gelatine, when determined in Lovibond units by the method described in Appendix P, shall be equal to that of an agreed sample. |
||||||||||||||
|
CLARITY |
||||||||||||||
|
18. The clarity of the gelatine, when determined by the method described in Appendix Q, shall be not less than that of an agreed sample. |
||||||||||||||
|
METHOD OF SAMPLING |
||||||||||||||
|
19. Sample increments shall be taken from a number of containers as set out in Table 1. |
||||||||||||||
|
TABLE 1. |
||||||||||||||
|
||||||||||||||
|
In the case of containers of not less than 1 cwt. the sample increment drawn shall be not less than 2 lb. where there is only one container, and not less than 1 lb. from each container in other instances. In the case of a container of less than 1 cwt. the sample increment shall be proportionately less. |
||||||||||||||
|
Each sample increment shall consist of |
||||||||||||||
|
(a) in the case of sheet and cake gelatine, the whole or equal portions of not less than four sheets or cakes representative of the contents of the container ; |
||||||||||||||
|
(b) in the case of leaf gelatine wrapped in packets, packets selected at random ; |
||||||||||||||
|
(c) in the case of flaked and kibbled gelatine, portions representative of the contents of the container ; |
||||||||||||||
|
(d) in the case of powdered and granulated gelatine portions taken by means of a sampling tube or scoop from the top, middle and bottom of the container. |
||||||||||||||
|
Sample increments shall be placed in clean, dry, air-tight, non-absorbent containers where they shall remain until the final samples are taken. |
||||||||||||||
|
The final sample shall be obtained as follows :-- |
||||||||||||||
|
Sheet and cake gelatine. A representative piece shall be taken from each sheet or cake and the pieces shall be broken up smaller and reduced by mixing or quartering to obtain a sample weighing not less than 2 lb. |
||||||||||||||
|
Leaf gelatine. Appropriate sections shall be sheared from each packet and reduced by mixing and quartering, to obtain a sample weighing not less than 2 lb. |
||||||||||||||
|
Powdered, flaked, kibbled or granulated gelatine. The material shall be thoroughly mixed and a 2 lb. sample taken from the bulk. |
||||||||||||||
|
The final samples shall be packed in clean, dry, air-tight, non-absorbent containers. The containers shall be of such a size that they are nearly filled by the sample. Each container so filled shall be marked with the date of sampling, and with sufficient information to identify the sample. |
||||||||||||||
|
AGREED SAMPLE |
||||||||||||||
|
20. The agreed sample referred to in this specification shall be one and the same sample and shall comply with the requirements of Clauses 2-12 inclusive of this specification. The sample shall be packed in the manner described in Clause 19. |
||||||||||||||
|
Appendix A |
||||||||||||||
|
PREPARATION OF SAMPLES FOR TESTS |
||||||||||||||
|
Working Sample : The sample shall be ground to a grist of approximately 1/8 inch by hand in an iron mortar or mechanically by means of a laboratory disintegrator of a type which has been agreed to by the purchaser and vendor. In the case of gelatines such as leaf gelatine which cannot be broken in a mortar and where a laboratory disintegrator is not available the sheets shall be cut as finely as possible with scissors. The sample shall then be quartered to obtain a sample weighing 1 lb. which shall be kept in an air-tight container. This sample is hereinafter referred to as the working sample. |
||||||||||||||
|
True Sample : A separate sample shall be set aside before the material is ground. This separate sample shall be either powdered in a mortar or cut up with a scissors. The finely divided material shall be placed in an air-tight container. The operations shall be carried out as rapidly as possible and under conditions free from excessive humidity or dryness. This sample, which is hereinafter referred to as the true sample, shall be used to determine the moisture content by the method described in Appendix B. |
||||||||||||||
|
Use of Working Sample: The moisture contents of the working sample and of the true sample shall be compared by carrying out determinations on the samples at the same time by the method described in Appendix B. From the results the weight of working sample which contains the same weight of anhydrous gelatine as one gram of the true sample shall be calculated. All determinations in Appendices C, D, E, F, G, H, J, M, P and Q shall be returned on the basis of the true sample and the quantities of working sample taken for the various tests shall be suitably adjusted so that the weight of anhydrous gelatine used in the tests is the same as if the true sample had been used. |
||||||||||||||
|
Appendix B |
||||||||||||||
|
DETERMINATION OF MOISTURE CONTENT |
||||||||||||||
|
One g. of the true sample shall be weighed in a tared flat stainless steel dish, 70 mm. in diameter, 15 mm. high and weighing between 20 g. and 30 g. fitted with an aluminium cover to be used during cooling and weighing. Ten ml. of distilled water shall be added and the gelatine allowed to soak. The dish shall be placed on a water bath so that the gelatine is dissolved and a homogeneous solution obtained and shall be left there until most of the water has evaporated. The dish shall be transferred to an oven maintained at a temperature of 105° C. ± 0·2° and shall be kept there for two hours, during which time the oven door shall not be opened. A thermometer shall be placed with its bulb ½ in. above the centre of the dish and the 105° mark approximately 2 in. above the outside top of the oven. Heating shall be continued for half-hour periods until the weight is constant to within 1 mg. when the gelatine shall be considered dry. |
||||||||||||||
|
The loss in weight in grams multiplied by 100 shall be regarded as the moisture content of the gelatine. |
||||||||||||||
|
Appendix C |
||||||||||||||
|
METHOD FOR THE DETERMINATION OF pH VALUE |
||||||||||||||
|
A quantity of working sample equivalent to 1 g. of true sample shall be dissolved in a small quantity of warm recently boiled distilled water in a stoppered flask of chemically resistant glass and the volume made up to 100 ml. with recently boiled and cooled distilled water. After shaking and allowing to cool to room temperature the pH value of the solution shall be determined by a recognised method, e.g., the glass electrode. |
||||||||||||||
|
Appendix D |
||||||||||||||
|
DETERMINATION OF CONTENT OF SULPHUR DIOXIDE |
||||||||||||||
|
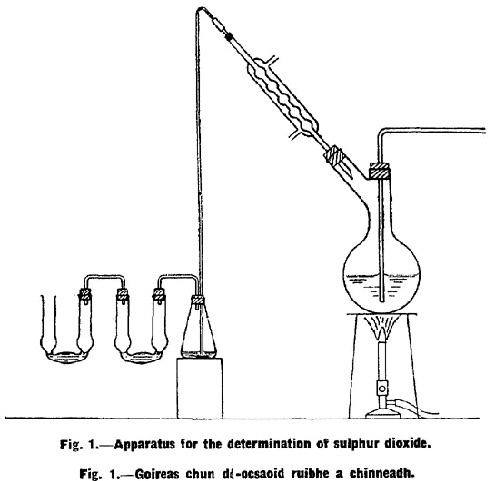
The apparatus shall consist of a 1,500 ml. round-bottomed flask with two necks, a reflux: condenser, a 200 ml. conical flask and two absorption tubes connected as shown in Fig. 1. One end of the inlet tube of the round-bottomed flask shall be within a ½ in. of the bottom of the flask, and the other shall be connected to a supply of carbon dioxide. All connections to flasks shall be made by glass tubing passing through rubber stoppers. Ten ml. of three per cent. neutral hydrogen peroxide solution free from sulphate shall be placed in the conical flask and the same quantity in the first absorption tube. The second absorption tube which serves as a guard shall contain 5 ml. of a mixture of hydrogen peroxide and barium chloride solution which has been slightly acidified with hydrochloric acid. |
||||||||||||||
|
Five hundred ml. of distilled water and 20 ml. of concentrated hydrochloric acid shall be placed in the round-bottomed flask and boiled for a short time in a current of carbon dioxide. The flask shall be allowed to cool while the flow of carbon dioxide is continued, and a quantity of working sample equivalent to 32 g. of the true sample shall be added to the flask by momentarily removing the stopper through which passes the lead-in tube. The flask shall be heated gently until the gelatine is dissolved. The contents of the flask shall then be boiled for one hour and during this period a slow current of carbon dioxide shall be passed through the flask. |
||||||||||||||
|
Immediately before the end of the distillation, the flow of water through the condenser shall be stopped to allow any sulphur dioxide which is retained by the condensed moisture in the tube of the condenser to be driven over into the receiver. Meanwhile, the receiver shall be cooled by immersing it in a vessel of water. As soon as the exit tube from the condenser is hot to the touch at the point of entry into the flask, the condenser shall be disconnected and washed down into the receiver with distilled water. The contents of the first absorption tube shall be added to the liquid in the conical flask and the liquid shall be titrated cold with N/10 sodium hydroxide solution using bromophenol blue as indicator. |
||||||||||||||
|
The content of sulphur dioxide shall be calculated. For 32 g. true sample 1 ml. N/10 NaOH is equivalent to 0·010 per cent. SO2, i.e., 100 parts per million. |
||||||||||||||
|
The solution in the second absorption tube should remain perfectly clear. If any precipitate of barium sulphate is formed, the test shall be repeated with an increased volume of hydrogen peroxide present in the conical flask and in the first absorption tube. |
||||||||||||||
|
Appendix E |
||||||||||||||
|
METHOD FOR THE DETERMINATION OF ASH |
||||||||||||||
|
A quantity of working sample equivalent to 5 g. of the true sample shall be incinerated in a platinum or silica basin over a low flame. When all the organic matter has become charred, the flame shall be slightly increased. When all the carbon has been burned, the basin shall be cooled in a desiccator and the weight of ash determined. If the sample is difficult to ash completely it shall be ashed until black and extracted with water. The residual carbon shall be ashed and the water extract added and evaporated to dryness. When igniting over a gas flame, after the gelatine has carbonised the residue should be pressed down on to the bottom of the crucible by a glass rod or agate pestle. |
||||||||||||||
|
It is essential to ash at as low a temperature as possible as many gelatines contain chlorides which are easily volatilised. Overheating shall be checked by carrying out chloride tests on the ash and on the original sample. |
||||||||||||||
|
Appendix F |
||||||||||||||
|
METHOD FOR THE DETERMINATION OF ARSENIC |
||||||||||||||
|
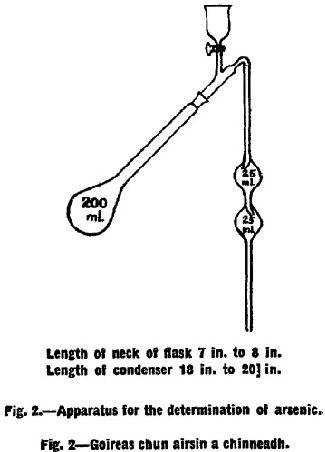
Preparation of sample for Gutzeit test. A quantity of working sample equivalent to 6 g. of the true sample shall be placed in a 200 ml. pyrex Kjeldahl flask with ground glass neck of the type shown in Fig. 2 and 10 ml. of concentrated nitric acid added. The flask shall be warmed gently over a bunsen flame until the evolution of nitrous fumes slackens and solution is complete. The flask shall be well cooled and 10 ml. of concentrated sulphuric acid added and gentle heating continued until darkening of the liquid sets in. While continuing the heating, concentrated nitric acid shall be added slowly from a dropping funnel at such a rate as will check the darkening of the liquid. It is most important that the mixture should be fully oxidised during the whole course of this operation in order to avoid loss of arsenious oxide. When the liquid becomes clear the heating shall be increased until white fumes appear and the liquid becomes colourless or pale yellow. It should not be necessary to use more than 35 ml. nitric acid in all. A persistent yellow colour in the liquid may be discharged by the addition of a few drops of 30 per cent. w/w solution of hydrogen peroxide and reheating. The liquid shall be cooled and 15 ml. of saturated ammonium oxalate solution added and the flask reheated until white fumes appear again. The residue shall be diluted with 7 ml. of water and cooled. |
||||||||||||||
|
A mixture of sodium chloride, hydrazine sulphate and potassium bromide in the proportions 5·0 to 0·5 to 0·02 respectively shall be prepared. This mixture maybe made up in bulk and kept in a stoppered bottle. Five g. of the chloride-hydrazine-bromide mixture shall be added to the flask through a short-stemmed funnel avoiding contamination of the ground portion of the neck of the flask. The glass condenser shown in Fig. 2 shall be fitted to the flask and 10 ml. of concentrated hydrochloric acid shall be added through the tap funnel fitted to the condenser. The liquid shall be distilled into an externally cooled mixture of 10 ml. water and 2 ml. nitric acid, the exit end of the condenser dipping below the surface of the liquid. |
||||||||||||||
|
The distillation shall be continued until the volume in the Kjeldahl flask is reduced by about half. The distillate shall be evaporated to dryness on a water bath, and the residue twice evaporated to dryness with 5 ml. water to remove nitric acid. The residue shall be dissolved in 3 ml. of warm concentrated sulphuric acid and diluted to 25 ml. with water. This solution shall be reduced with zinc in the Gutzeit apparatus as follows. |
||||||||||||||
|
Operation of the Gutzeit apparatus. The apparatus shall consist of a wide-mouth bottle, holding about 120 ml., fitted with a rubber bung through which passes a glass tube. The glass tube shall be made from ordinary glass tubing and shall have a total length of 200 mm., an internal diameter of exactly 6·5 mm., an external diameter of about 8 mm. It shall be drawn out at one end to a diameter of about 1 mm., and a hole not less than 2 mm. in diameter shall be blown in the side of the tube near the constricted part. The tube shall be inserted in the bung so that when the bung is fitted to the bottle containing 60 ml. of liquid, the constricted end of the tube is above the surface of the liquid and the hole in the side is below the bottom of the bung. The upper end of the tube shall be cut off square and either slightly rounded off or ground smooth. |
||||||||||||||
|
Prior to each determination, a strip of lead acetate paper rolled up to form a cylinder 100 mm. in length shall be placed in the glass tube, so that the upper end is not less than 25 mm. below the top of the tube. The lead acetate papers shall be prepared by soaking strips of white filter paper 100 mm. x 50 mm. in a solution of 20 g. of normal lead acetate dissolved in 100 ml. of water and rendered just acid to litmus paper by the addition of acetic acid. The surplus solution shall be removed from the soaked paper by pressing between filter papers and then drying the paper at a temperature not exceeding 37°C. |
||||||||||||||
|
Mercuric bromide test papers shall be prepared by soaking filter paper, similar in substance and texture to No. 130 Whatman filter paper, in a 5 per cent. alcoholic solution of mercuric bromide, the surplus solution being removed by pressing between filter papers and the test paper allowed to air-dry in the dark. The edges of the dry papers shall be removed and the papers shall be stored in a stoppered bottle in the dark. |
||||||||||||||
|
The test papers used shall be in the form of a disc and shall be held flatly and firmly against the ground end of the tube by any suitable means, provided that the whole of the evolved gas passes through the paper and, that the portion in contact with the gas is a circle 6·5 mm. in diameter. Securing the test papers with rubber bands is not regarded as a satisfactory method. |
||||||||||||||
|
In carrying out the test, either the whole of the solution, or if the arsenic content is more than 0·5 parts per million, such an aliquot portion as would contain not more than 0·003 mg. arsenic shall be mixed in the apparatus with sulphuric acid, hydrochloric acid and water in the following proportions :-- |
||||||||||||||
|
z ml. of prepared solution, |
||||||||||||||
|
25 minus z ml. of 1 : 8 sulphuric acid (by volume), |
||||||||||||||
|
8 ml. of hydrochloric acid and water to make up to 60 ml. |
||||||||||||||
|
Before proceeding to the actual Gutzeit determination all the arsenic shall be reduced to the tervalent form by adding to the mixture 1 ml. of 10 per cent. potassium iodide solution, and 0·5 ml. stannous chloride (B.P., As. T.) solution and then heating the mixture to 85°C. and maintaining it at 85°C. to 90°C. for 10 minutes and then cooling to room temperature. |
||||||||||||||
|
Ten g. of granulated zinc shall then be added and the apparatus immediately assembled. |
||||||||||||||
|
The reaction shall be allowed to proceed at room temperature for two hours, the mercuric bromide papers being protected from strong sunlight during the reaction. The stain so produced on the paper shall be compared with a series of freshly made standard stains prepared by putting known amounts of arsenic through the full process described above, the stains being examined preferably under normal daylight conditions. Complete " blank " tests shall be made to check all the reagents and apparatus used in the test. |
||||||||||||||
|
Arsenic solution for the preparation of standard stains shall be prepared as follows :-- |
||||||||||||||
|
Strong solution of Arsenic, 0·132 g. of arsenic trioxide dissolved in 50 ml. hydrochloric acid and diluted to 100 ml. with water. |
||||||||||||||
|
Dilute solution of Arsenic, 1 ml. of above solution diluted to 100 ml. with water. This solution contains 0·01 mg. arsenic per ml. The solution does not keep and should be made up freshly each week. |
||||||||||||||
|
Appendix G |
||||||||||||||
|
METHOD FOR THE DETERMINATION OF HEAVY METALS |
||||||||||||||
|
Preparation of Solution: A quantity of working sample equivalent to 20 g. of the true sample shall be incinerated in a clean platinum or porcelain dish over a low flame. When all the organic matter has become charred the flame shall be slightly increased. Heating shall be continued until a white ash is obtained. This incineration process calls for particular care, because too high a temperature will cause loss of metals by volatilisation whereas too low a temperature will cause errors in the subsequent tests due to colouring of the solutions with carbon. Five ml. of concentrated hydrochloric acid shall be added to the ash and the mixture evaporated to dryness on a water-bath. The residue shall be dissolved in a mixture of 5 ml. concentrated hydrochloric acid and 20 ml. of water and the solution filtered. To the filtrate and washings 2 g. of pure citric acid and 10 mg. of crystallised ferrous sulphate shall be added. |
||||||||||||||
|
The amount of heavy metals in the reagents used shall be determined and appropriate corrections applied to the results of the tests. |
||||||||||||||
|
Separation 1. To the filtrate which should amount to about 40 ml., three drops of Universal Indicator shall be added, and then ammonium hydroxide until the cold solution shows a green colour, indicating a pH value of about 8. The wrong pH is obtained if the final adjustment is made while the solution is hot. Hydrogen sulphide shall be passed into the solution for 20 to 30 minutes, and the black precipitate shall be filtered off through a small No. 2 Whatman paper. The precipitate shall be washed well with a solution of hydrogen sulphide made alkaline with ammonium hydroxide, and then with water until it is free from ammonium citrate. |
||||||||||||||
|
Separation 2. The filter paper containing the black sulphide precipitate shall be transferred to a beaker containing 25 ml. of water and 2 ml. of concentrated nitric acid and heated on a hot-plate. After the sulphides have dissolved the filter paper shall be removed with a glass rod and replaced in the funnel, but should the filter paper become " pulped " during the boiling, the solution shall be filtered through a new small filter paper, and the combined pulp and filter paper washed with hot water. The nitric acid solution shall be filtered through it and the filter well washed with hot water. To the filtrate, which should be between 40 and 50 ml. in volume, 1 g. of pure ammonium sulphate shall be added and the solution heated until it is near the boiling point. The iron shall be precipitated by the addition of 15 ml. of 15 per cent. w/v ammonium hydroxide solution, and the heating continued until the ferric hydroxide has coagulated. The solution shall not be boiled for a long time during this precipitation since there is the possibility that most of the ammonia may be evolved and absorption of the copper that is present may take place. The precipitated ferric hydroxide shall be filtered off and washed with hot water and 2 per cent. ammonium hydroxide solution. |
||||||||||||||
|
Separation 3. Determination of Lead. The filter paper containing the ammonium hydroxide precipitate shall be transferred to a beaker containing 20 ml. of water and 2 ml. of concentrated nitric acid and heated on a hot-plate. After the precipitate has dissolved the filter shall be removed with a glass rod and replaced in the funnel. The nitric acid solution shall be filtered through it and the filter washed well with hot water. The filtrate shall be transferred to a separating funnel and, when cold, 5 ml. of a saturated ammonium thiocyanate solution shall be added. Fifteen ml. of amyl alcohol and 15 ml. of ether shall be added and the whole vigorously shaken. When the two layers have separated, the aqueous layer shall be drawn off, and the extraction shall be carried out a second time with 10 ml. of ether and 10 ml. of amyl alcohol. The aqueous layer shall be drawn off once more and 1 ml. of a 10 per cent. potassium cyanide solution added and the solution made alkaline with ammonium hydroxide. |
||||||||||||||
|
The solution shall be transferred to a Nessler glass conforming to B.S. 612 : 1935 and two drops of a 10 per cent. solution of sodium sulphide added and then distilled water to bring the volume of solution to the 50 ml. mark and the solution shall then be shaken. The colour of the solution shall be matched against that of a solution in a similar Nessler glass, the solution consisting of a mixture of water, 1 ml. of 10 per cent. potassium cyanide, 2 drops of 10 per cent. sodium sulphide solution and a known quantity of lead nitrate in solution. |
||||||||||||||
|
Separation 4. Determination of Copper and Zinc. The filtrate from the ammonium hydroxide precipitate of Separation 2 shall be made. up to 100 ml. Copper shall be determined in 20 ml. of this solution by means of sodium diethyl-dithio-carbamate. If copper is present it shall be removed before testing for zinc. |
||||||||||||||
|
The remaining 80 ml. of the solution shall be evaporated to a bulk of 20 ml. and 5 ml. of 4 N hydrochloric acid added and warmed to dissolve any traces of zinc that have been adsorbed on the glass during the evaporation. The solution shall be cooled and hydrogen sulphide passed into it for 15 minutes to precipitate the copper as sulphide. When only traces of copper are present, 1 mg. of copper as copper sulphate may be added before passing the hydrogen sulphide in order to facilitate the coagulation and filtration of the copper. The precipitated copper sulphide shall be filtered off and washed with hydrogen sulphide water. The filtrate thus obtained shall be evaporated to a bulk of 20 ml. The zinc in the solution shall be determined as follows : |
||||||||||||||
|
Five ml. of 20 per cent. ammonium acetate solution and 2 drops of Universal Indicator shall be added and the pH of the solution adjusted to 7·5 by the addition of ammonium hydroxide. Then 2 ml. of 2 per cent. 8-hydroxyquinoline in N acetic acid shall be added and the solution boiled for 5 minutes in a covered beaker. After boiling, the beaker shall be allowed to stand for 5 minutes and the precipitate filtered off and washed well with hot water. The precipitate shall be dissolved in hydrochloric acid by washing the filter twice with 3 N hydrochloric acid and then twice with water, the total volume of the washings which are collected in a stoppered bottle, being about 30 ml. To this solution shall be added N/50 bromide and bromate solution until a drop of the solution yields a reaction with starch-iodide paper, then 2 ml. excess shall be added and the solution shaken well. After standing for 5 minutes in the dark, a few crystals of potassium iodide shall be added, and the excess bromine determined by titration with N/50 sodium thiosulphate solution. The N/50 bromide and bromate solution shall be prepared by dissolving 0·554 g. potassium bromate and 2·24 g. potassium bromide in 1 litre of distilled water, and standdardising against N/50 sodium thiosulphate solution. |
||||||||||||||
|
Appendix H |
||||||||||||||
|
METHOD FOR THE DETERMINATION OF TOTAL BACTERIOLOGICAL COUNT |
||||||||||||||
|
A quantity of working sample equivalent to 5 g. of the true sample shall be weighed and ice-cold water added to make the total volume 100 ml. The whole shall be allowed to stand for 2 hours and then kept in a water-bath at 50°C. for 15 minutes and then well shaken. Twenty ml. of the solution shall be diluted with 80 ml. of sterile distilled water thus producing a solution of 1 in 100 dilution. One ml. of the 1 in 100 solution shall be placed in a sterile petri-dish using a sterile 1 ml. pipette and 15 ml. of liquefied nutrient agar at 46°C. shall be added. The plate shall be incubated at 37°C. for 48 hours. |
||||||||||||||
|
The number of colonies on the plate shall be counted and the number of organisms per gram of gelatine calculated. |
||||||||||||||
|
Appendix J |
||||||||||||||
|
METHOD FOR THE DETERMINATION OF JELLY STRENGTH BY THE BLOOM GELOMETER |
||||||||||||||
|
The test bottle used shall be an extra wide-mouthed one taking a rubber stopper of diameter 42-45 mm. It shall have a capacity of 155 ml. and an overall height of 85 mm. The outside diameter shall be 66 mm. and the average internal diameter shall not vary by more than 1 mm. from 59 mm. |
||||||||||||||
|
The stopper used shall be tapered and shall be cut in half and the upper portion perforated by plunging a red hot, 1 inch brad through it at the centre. The upper half of the stopper shall be used to obtain a snug fit in the neck of the bottle, the air vent serving to prevent the stopper from being blown out during the melting and heating of the sample. The test bottle and stopper shall be clean and dry. |
||||||||||||||
|
A quantity of gelatine equivalent to 7·5 g. of the true sample shall be weighed and transferred to the test bottle. Then 105 ml. of distilled water at approximately 15°C. shall be added while thoroughly stirring the gelatine with a thin metal rod. |
||||||||||||||
|
The bottle shall be placed in a cooler maintained at a temperature of 10°C. to 15°C. and the sample allowed to soak for three hours. |
||||||||||||||
|
To prevent cracking, and in order to preserve uniformity in heating and cooling, the following procedure shall be adopted both when heating the sample to 60°C. and, after solution, when cooling prior to putting in a bath. Place the bottle for 15 minutes in a water bath kept at approximately 30°C. Bring the sample to a temperature of 60°C. in the melting bath, the temperature of which is not allowed to exceed 70°C. Determine the temperature of the sample with an accurate thermometer placed in the gelatine solution and carried by a stopper having a small perforation off the centre for this purpose. |
||||||||||||||
|
The time required to bring the sample up to temperature shall not exceed 15 minutes. After closing the bottle with the stopper carrying the thermometer and before reaching the final temperature the solution shall be made thoroughly uniform preferably by swirling the bottle a number of times, but avoiding any motion that will produce violent agitation of the solution. |
||||||||||||||
|
The finger shall be placed over the perforation in the stopper and the test bottle inverted several times to mix in the water that has condensed on the walls of the bottle and the under side of the stopper. Then the container shall be placed in a totally enclosed chill bath maintained at a temperature of 10°C. ± 0·1° for not less than 16 nor more than 18 hours. |
||||||||||||||
|
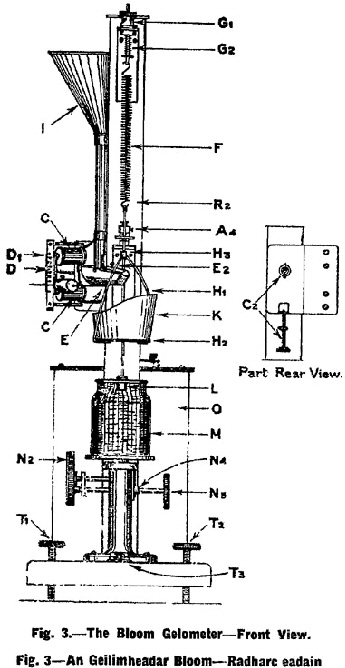
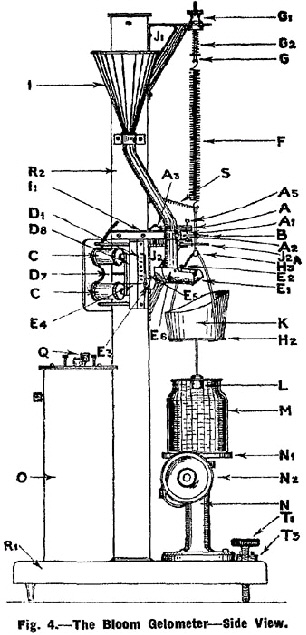
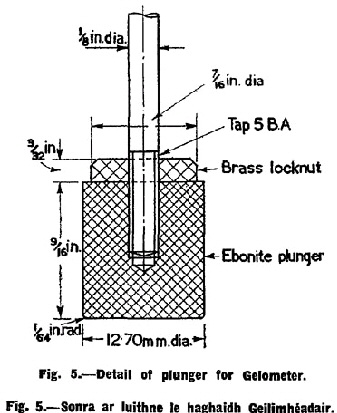
The determination of the jelly strength of the sample shall be made with the Bloom Gelometer (See Fig. 3, Fig. 4 and Fig. 5) adjusted to give a 4 mm. depression, and to deliver shot at the rate of 200 g. per 5 seconds, when the clam-shell arm E4 rests upon dog D1 and 800 g. of polished No. 12 chilled lead shot are in hopper 1. |
||||||||||||||
|
The Bloom Gelometer shall be placed perfectly level on a rigid support. It shall be levelled by turning thumb-screw nut G1 until the silver disc is well above the lower contact point A2, and by means of levelling screws T1 and T2 the base R1 shall be adjusted so that rod H3 hangs in the centre of the hole in guide arm J2A. Brass contact point bracket A shall be set so that it will not come into contact with rod H3, nor shall the former be allowed to come in contact with screws holding J2A in position, otherwise a " short " in the circuit will result. The position of J2A shall not be changed as it must be kept directly under the point of suspension of spring F. |
||||||||||||||
|
The spring shall be of such a stiffness that between 2 and 3 grams placed on the pan H2 will bring the disc B from top contact A1 to lower contact A2. |
||||||||||||||
|
The weight of the pan system shall be counter-balanced by such a tension in the spring F that equilibrium is produced when contact disc B is just barely resting on the lower contact point A2. This condition shall be produced by first closing the electrical circuit by means of switch Q and then turning thumbscrew nut G1 until disc B on lowering makes the first perceptible electrical contact with lower contact point A2. When properly set, a slight vibration of the instrument will cause a succession of " makes " and " breaks " in the circuit producing a sound in mechanism D1, D7, D8 very much like that of a telegraph sounder. |
||||||||||||||
|
To make a test of the jelly strength of a given sample, the electrical circuit shall be closed by means of switch Q the jelly contained in the test bottle M shall be centred on platform N1 and by means of the rack and pinion elevating mechanism N2 the jelly shall be raised until disc B almost makes contact with contact point A1. Then with the fine adjustment on rack and pinion N3 the disc shall be brought into the lightest, but positive, electrical contact with contact point A1. This is indicated by sparking and the telegraph sounder effect noted above. The shot receiver K shall be quickly placed on pan H2 and immediately the arm E4 shall be raised to the predetermined position on one of the dogs D1 with a quick but uniform motion. The height to which arm E4 is raised regulates the velocity of the flow of shot, plunger L depressing the surface of the jelly until disc B makes contact with contact point A2. This closes the circuit which acts on the electromagnet C, moving the soft iron bar D8 and withdrawing the support of the dog from the arm E4, which immediately falls, thus cutting off the flow of shot by closing the clam-shell cut-off E-E1. |
||||||||||||||
|
All determinations shall be made from the dog D1 and the results expressed to the nearest whole gram. |
||||||||||||||
|
The weight of shot delivered into the receiver K plus the weight of the shot receiver itself is the weight required to move the plunger L through a distance of 4 mm. against the resistance of the jelly and, for the purpose of Clause 13, this weight shall be the Bloom jelly strength of the sample. |
||||||||||||||
|
Appendix K |
||||||||||||||
|
COMPARISON OF JELLY STRENGTH OF A SAMPLE AGAINST AN AGREED SAMPLE |
||||||||||||||
|
Equal weights of the working sample and of the agreed sample, prepared in the same way as the working sample shall each be weighed and placed in two 150 ml. beakers and 50 ml. of cold distilled water shall be added to each from a pipette. The quantity of gelatine used (2 to 10 g.), the concentration, and the temperature at which the solutions are made, shall be the same in both cases and shall be the subject of agreement between the purchaser and vendor. |
||||||||||||||
|
A watch glass shall be placed over each beaker and the gelatine allowed to soak for about three hours until it is completely swollen. During soaking, the temperature of the room shall be from 15°C. to 20°C. Each beaker shall then be heated on a water bath for 15 minutes while the contents are gently stirred with a glass rod. The temperature of the water bath shall be not more than 70°C. and the temperature of the gelatine solution shall be not more than 60°C. If necessary the heating shall be continued until, on inspection through the bottom of the beaker, the gelatine appears to be completely dissolved. |
||||||||||||||
|
Each of the gelatine solutions shall be immediately poured into aluminium or porcelain cups. The cups shall be of exactly the same internal dimensions. After two minutes a lid shall be placed on each vessel. The cups shall be left to stand for 16 hours in a thermostatic bath maintained at a temperature agreed to by the purchaser and vendor. |
||||||||||||||
|
The lids shall be removed from the vessels and the two jellies compared by pressure with the finger or by any suitable instrument or procedure agreed to by the purchaser and vendor. |
||||||||||||||
|
Appendix L |
||||||||||||||
|
METHOD FOR THE DETERMINATION OF THE SOLUBILITY OF THE PARTIALLY SWOLLEN SHEET |
||||||||||||||
|
One lb. of the sheet gelatine shall be coarsely crushed with an iron pestle and mortar to pass a 3/8 in. test sieve conforming to I.S. 24 : 1950. Twenty ml. of water at 27°C. shall be measured into a small basin and 20 g. of the crushed gelatine shall be added at once. The gelatine shall be stirred immediately and continuously with a suitable rod until all the water has been absorbed. The soaked gelatine shall be left to mature in a closed vessel containing water so that it is subjected to an atmosphere of 95 to 100 per cent. relative humidity for 24 hours at room temperature. Five hundred ml. of water at 60°C. shall be placed in a beaker and the swollen gelatine added. The contents shall be stirred gently with a thermometer for 4 minutes, the temperature being maintained at 60°C., and then poured through a No. 200 test sieve conforming to I.S. 24 : 1950. The residue on the sieve shall be rinsed with cold water and transferred to a weighed stainless steel dish and then dried in an oven at 105°C. to constant weight. The stainless steel dish shall be as specified for the determination of moisture content in Appendix B. The weight so obtained shall be divided by 0·85 to allow for moisture content and the corrected figure shall be multiplied by 5. The result shall be expressed as per cent. insoluble residue of sheet gelatine swollen at 1 : 1. The figure obtained by subtracting the result from 100 shall be taken as the solubility of the partially swollen sheet. |
||||||||||||||
|
Appendix M |
||||||||||||||
|
METHOD FOR THE DETERMINATION OF MELTING POINTy |
||||||||||||||
|
A quantity of working sample equivalent to 7·5 g. of the true sample shall be placed in a beaker and 105 ml. of cold distilled water added. A watch glass shall be placed over the beaker and the gelatine allowed to soak for about three hours until it is completely swollen. During soaking the temperature of the room shall be from 15°C. to 20°C. The beaker shall then be heated on a water bath for 15 minutes while the contents are gently stirred with a glass rod. The temperature of the water bath shall be not more than 70°C. and the temperature of the gelatine solution shall be not more than 60°C. If necessary the heating shall be continued until, on inspection through the bottom of the beaker, the gelatine appears to be completely dissolved. |
||||||||||||||
|
The apparatus for determination of melting point shall consist of a brass bowl 22 mm. in height, 17 mm. external diameter at the top, and 15 mm. external diameter at the bottom, and of such thickness that its weight is 7·00 g. Into this shall be loosely fitted a glass rod 40 mm. long and 3 mm. in diameter which is flattened at one end to a disc of 9 mm. diameter and fashioned at the other end into a hook. |
||||||||||||||
|
The gelatine solution shall be poured into the bowl, and the rod placed in a vertical position in the bowl with the disc resting on the base of the bowl. The bowl with the rod maintained in a vertical position shall be kept for 16 hours in a thermostat at 10°C. to allow the solution to set to a jelly. The apparatus shall be suspended in a beaker of water at 15°C. so that it is completely immersed. The beaker of water with the apparatus shall then be placed in a water bath at 20°C. and the water in the water bath heated so that the temperature of the water in the beaker rises at a rate of ¼°C. per minute. |
||||||||||||||
|
The temperature of the water in the beaker at which the bowl falls from the rod shall be taken as the melting point of the gelatine. |
||||||||||||||
|
The melting point of the agreed sample shall be determined by treating an equal quantity in the same way. |
||||||||||||||
|
Appendix N |
||||||||||||||
|
METHOD FOR THE DETERMINATION OF WATER ABSORBTION |
||||||||||||||
|
The working sample shall be sieved and the portion passing through a No. 22 test sieve, conforming to I.S. 24 : 1950, and retained on a No. 44 test sieve, conforming to I.S. 24 : 1950, shall be used for the test. If the original sample is of such a grist that it passes through a No. 44 test sieve, it shall be used without further treatment. |
||||||||||||||
|
Seven and a half g. of the powder so obtained shall be added to 210 ml. distilled water at 10°C. in a 300 ml. squat beaker. The contents shall be occasionally stirred with a glass rod at the earlier stages to prevent caking. The beaker and contents shall be kept at 10°C. for 16 hours. The supernatant water shall be poured off through a funnel fitted with a strainer of stretched damp muslin. The quantity of water passing through the funnel, allowing a drainage time of five minutes, shall be measured. The difference between this figure and 210 shall be taken as the water absorbed by the 7·5 g. of powder. |
||||||||||||||
|
The water absorbed by 7·5 g. of the agreed sample shall be determined in the same way. |
||||||||||||||
|
Appendix P |
||||||||||||||
|
METHOD FOR THE DETERMINATION OF COLOUR |
||||||||||||||
|
The colour shall be expressed in Lovibond units for a 6·66 per cent w/w gel in a 2 in. cell, after keeping for 2 hours in a thermostatically controlled water bath at 15°C. |
||||||||||||||
|
A quantity of working sample equivalent to 7·5 g. of the true sample shall be soaked and dissolved as described in Appendix M. The solution shall be poured into a 2 in. Lovibond cell and the colour determination made in a standard Lovibond Tintometer using the artificial light attachment supplied with the instrument and any or all of the following seven slides : |
||||||||||||||
|
1 slide containing tens and twenties of red, yellow and blue, |
||||||||||||||
|
1 red slide, 1 yellow slide, and 1 blue slide each containing 1 to 9. |
||||||||||||||
|
1 red slide, 1 yellow slide, and 1 blue slide each containing 0·1 to 0·9. |
||||||||||||||
|
The actual readings obtained for yellow, red and blue shall be recorded. Should the reading for any one colour be more than 15 units, a fresh determination shall be made in a smaller cell. |
||||||||||||||
|
The colour of the agreed sample shall be determined by treating an equal quantity in the same way. |
||||||||||||||
|
Appendix Q |
||||||||||||||
|
METHOD FOR THE DETERMINATION OF CLARITY |
||||||||||||||
|
The light transmitted through a 6·66 per cent. w/w gelatine solution contained in a 2 in. glass-ended trough shall be measured photoelectrically and expressed as a percentage of the light transmitted through distilled water under the same conditions, as described below. This percentage shall be taken as the measure of the clarity of the solution. |
||||||||||||||
|
A quantity of working sample equivalent to 7·5 g. of the true sample shall be soaked and dissolved as described in Appendix M. It shall be poured into a 2 in. Lovibond silvered cell, and kept in a water bath at 15°C. for 2 hours before testing. |
||||||||||||||
|
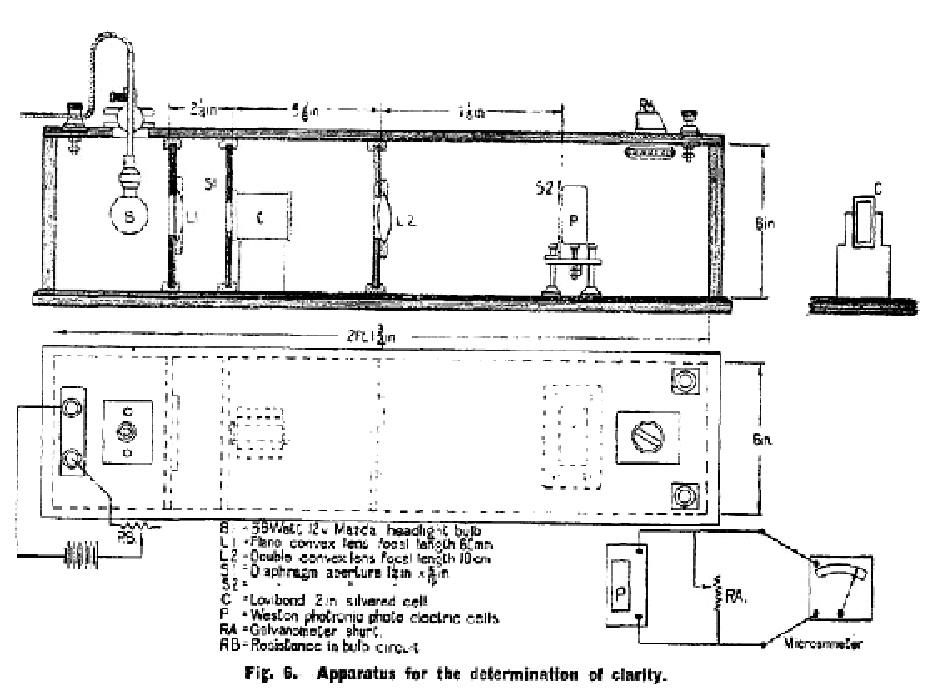
The instrument used for carrying out this test (see Fig. 6) shall consist essentially of a Weston photronic photoelectric cell connected to a microammeter of low internal resistance (not greater than 100 ohms). The sensitive face of this cell shall be illuminated by a parallel beam of light from a source the intensity of which is adjusted so that when the Lovibond cell containing distilled water is placed in the path of the beam a reading of 100 m.a. is obtained on the microammeter. The current recorded when the Lovibond cell containing the gelatine solution is substituted shall be taken as a measure of the clarity of the sample. |
||||||||||||||
|
The light source shall be a 12 volt 36 watt Mazda head lamp bulb placed at the focus of a plano-convex lens (2 in. diameter, focal length, 60 mm.). The current shall be supplied by an accumulator. In front of the Lovibond cell shall be placed a diaphragm with a rectangular aperture, size 1¼ in. x 5/16 in., designed to reduce reflection effects from the walls of the cell and from the meniscus of the gelatine solution. The light emerging from the Lovibond cell shall be collected and condensed by a double convex lens (2 in. diameter focal length 10 cm.) and received on the photoelectric cell fitted with a diaphragm of similar aperture to the first. The lens shall be placed approximately 15 cm. away from the photronic cell so that the image is only slightly larger than the aperture in the diaphragm. Adjustments in the reading on the microammeter may be made by altering (a) a resistance in the lamp circuit (a coarse adjustment) (b) a shunt on the galvanometer (a fine adjustment). |
||||||||||||||
|
A reading with a Lovibond cell containing distilled water shall be taken before every measurement on a gelatine sample to eliminate the effect of any variation in the sensitivity of the cell or in the voltage across the bulb. |
||||||||||||||
|
GIVEN under my Official Seal this 9th day of February, 1953. |
||||||||||||||
|
(Signed) SEÁN F. LEMASS, |
||||||||||||||
|
Minister for Industry and Commerce. |
||||||||||||||
|
_______________________________________________________________________ |
||||||||||||||
|
ACKNOWLEDGEMENT |
||||||||||||||
|
The method for the determination of jelly strength by the Bloom Gelometer given in Appendix J is based on a method published in " Industrial and Engineering Chemistry ", Analytical Edition, 2,348 (1930). The method was originally adopted by the National Association of Glue Manufacturers, Atlantic City, New Jersey, October 10, 1923, and published in " Industrial and Engineering Chemistry ", Volume 16, 310 (1924). |
||||||||||||||
|
Figures 1, 3, 4, 5 and 6 of B.S. 757 : 1944 " Methods for sampling and testing gelatines " are included by permission of the British Standards Institution, 24/28, Victoria Street, London, S.W.I., who reserve all copyright. |
||||||||||||||
|
|
||||||||||||||
|
|
||||||||||||||
|
" |
||||||||||||||
|
Details of Fig. 3 (Front View). |
||||||||||||||
|
A1. Set screw to hold adjustment screw in position. |
||||||||||||||
|
C. Electromagnet. |
||||||||||||||
|
C2. Adjustment screw for adjusting pitch of clam cut-off E. |
||||||||||||||
|
D. Guide bar of automatic shot control mechanism. |
||||||||||||||
|
D1. Dog. |
||||||||||||||
|
E. Clam-shell spout. |
||||||||||||||
|
E2 Adjusting screws to regulate closure. |
||||||||||||||
|
F. Spiral spring (No. 6 steel music wire). |
||||||||||||||
|
G1. Thumb screw nut. |
||||||||||||||
|
G2. Tension spring. |
||||||||||||||
|
H1. Pan arms. |
||||||||||||||
|
H2. Pan |
||||||||||||||
|
H3. Rod attached to pan arms supporting disc. B. |
||||||||||||||
|
I. Shot hopper with delivery tube. |
||||||||||||||
|
K. Short receiver. |
||||||||||||||
|
L. Plunger (12.7 mm in diameter). |
||||||||||||||
|
M. Test bottle. |
||||||||||||||
|
N2. Rack and pinion elevating mechanism. |
||||||||||||||
|
N3. Fine adjustment on rack and pinion. |
||||||||||||||
|
N4. Brake shoe on adjustment arm of N3. |
||||||||||||||
|
O. Battery box and batteries. |
||||||||||||||
|
R2. Pillar of gelometer. |
||||||||||||||
|
T1, T2. Levelling screws. |
||||||||||||||
|
T3. Spirit level. |
||||||||||||||
|
|
||||||||||||||
|
Details of Fig. 4 (Side View). |
||||||||||||||
|
A Brass contact point bracket. |
||||||||||||||
|
A1. Upper contact point. |
||||||||||||||
|
A2. Lower contact point. |
||||||||||||||
|
A3. Wood fibre support for A. |
||||||||||||||
|
A5. Adjustment screw. |
||||||||||||||
|
B. Pure silver disc (5/8 inch dia. and 1/16 inch thick). |
||||||||||||||
|
C. Electromagnet. |
||||||||||||||
|
D1. Dog. |
||||||||||||||
|
D7. Hair spring coil to keep D8 in position. |
||||||||||||||
|
D8. Soft iron bar supporting dog D1. |
||||||||||||||
|
E1. Stationary clam-shell jaw. |
||||||||||||||
|
E2. Adjusting screws to regulate closure. |
||||||||||||||
|
E3. Weight. |
||||||||||||||
|
E4. Clam-shell arm. |
||||||||||||||
|
E5. Set screw to clamp weight to clam-shell arm. |
||||||||||||||
|
E6. Bearing on which cut-off mechanism turns. |
||||||||||||||
|
F. Spiral spring (No. 6 steel music wire). |
||||||||||||||
|
G. Adjustable support for spring F. |
||||||||||||||
|
G1. Thumb-screw nut. |
||||||||||||||
|
G2.Tension spring. |
||||||||||||||
|
H2. Pan. |
||||||||||||||
|
H3. Rod attached to pan arms supporting disc B. |
||||||||||||||
|
I. Shot hopper with delivery tube. |
||||||||||||||
|
I1. Bracket to hold lower end of shot delivery tube. |
||||||||||||||
|
J1. Upper supporting bracket attached to frame support R2. |
||||||||||||||
|
J2. Lower supporting bracket attached to frame support R2. |
||||||||||||||
|
J2A. Guide arm attached to J2. |
||||||||||||||
|
K. Shot receiver. |
||||||||||||||
|
L. Plunger (bevelled typre). The dimensions of the plunger are shown in Fig. 5. |
||||||||||||||
|
M. Test bottle. |
||||||||||||||
|
N. Elevating platform base. |
||||||||||||||
|
N1. Platform. |
||||||||||||||
|
N2. Rack and pinion elevating mechanism. |
||||||||||||||
|
O. Battery box and batteries. |
||||||||||||||
|
Q. Electrical switch. |
||||||||||||||
|
R1. Base of gelometer. |
||||||||||||||
|
R2. Pillar of gelometer. |
||||||||||||||
|
S. Fine copper wire coil making contact across from suspended disc to binding past on support. |
||||||||||||||
|
T1. Levelling screws. |
||||||||||||||
|
T8. Spirit level. |
||||||||||||||
|
|
||||||||||||||
|
|
||||||||||||||
|
|